MEHP
General
Type : Phthalate
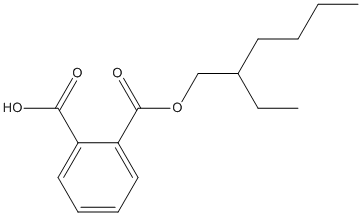
Chemical_Nomenclature : 2-(2-ethylhexoxycarbonyl)benzoic acid
Canonical SMILES : CCCCC(CC)COC(=O)C1=CC=CC=C1C(=O)O
InChI : InChI=1S\/C16H22O4\/c1-3-5-8-12(4-2)11-20-16(19)14-10-7-6-9-13(14)15(17)18\/h6-7,9-10,12H,3-5,8,11H2,1-2H3,(H,17,18)
InChIKey : DJDSLBVSSOQSLW-UHFFFAOYSA-N
Other name(s) : 4376-20-9, 2-(((2-Ethylhexyl)oxy)carbonyl)benzoic acid, Mehp, Mono(2-ethylhexyl) phthalate, Mono(2-ethylhexyl)phthalate, 2-Ethylhexyl hydrogen phthalate
MW : 278.34
Formula : C16H22O4
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Carbon-carbon_bond_hydrolase, AlphaBeta_hydrolase
References (5)
| Title : Mono-2-ethylhexylphthalate (MEHP) is a potent agonist of human TRPA1 channel - Goh_2023_Chemosphere__140740 |
| Author(s) : Goh M , Fu L , Seetoh WG , Koay A , Hua H , Tan SM , Tay SH , Jinfeng EC , Abdullah N , Ng SY , Lakshmanan M , Arumugam P |
| Ref : Chemosphere , :140740 , 2023 |
| Abstract : Goh_2023_Chemosphere__140740 |
| ESTHER : Goh_2023_Chemosphere__140740 |
| PubMedSearch : Goh_2023_Chemosphere__140740 |
| PubMedID: 38006918 |
| Title : Excellent Degradation Performance of a Versatile Phthalic Acid Esters-Degrading Bacterium and Catalytic Mechanism of Monoalkyl Phthalate Hydrolase - Fan_2018_Int.J.Mol.Sci_19_ |
| Author(s) : Fan S , Wang J , Yan Y , Jia Y |
| Ref : Int J Mol Sci , 19 : , 2018 |
| Abstract : Fan_2018_Int.J.Mol.Sci_19_ |
| ESTHER : Fan_2018_Int.J.Mol.Sci_19_ |
| PubMedSearch : Fan_2018_Int.J.Mol.Sci_19_ |
| PubMedID: 30231475 |
| Gene_locus related to this paper: 9acto-q2mhh5 |
| Title : Biodegradation of phthalic acid esters (PAEs) and in silico structural characterization of mono-2-ethylhexyl phthalate (MEHP) hydrolase on the basis of close structural homolog - Singh_2017_J.Hazard.Mater_338_11 |
| Author(s) : Singh N , Dalal V , Mahto JK , Kumar P |
| Ref : J Hazard Mater , 338 :11 , 2017 |
| Abstract : Singh_2017_J.Hazard.Mater_338_11 |
| ESTHER : Singh_2017_J.Hazard.Mater_338_11 |
| PubMedSearch : Singh_2017_J.Hazard.Mater_338_11 |
| PubMedID: 28531656 |
| Gene_locus related to this paper: 9psed-a0a1h9kym9 |
| Title : Mono-2-ethylhexyl phthalate inhibits human extravillous trophoblast invasion via the PPARgamma pathway - Gao_2017_Toxicol.Appl.Pharmacol_327_23 |
| Author(s) : Gao F , Hu W , Li Y , Shen H , Hu J |
| Ref : Toxicol Appl Pharmacol , 327 :23 , 2017 |
| Abstract : Gao_2017_Toxicol.Appl.Pharmacol_327_23 |
| ESTHER : Gao_2017_Toxicol.Appl.Pharmacol_327_23 |
| PubMedSearch : Gao_2017_Toxicol.Appl.Pharmacol_327_23 |
| PubMedID: 28416457 |
| Title : Re-characterization of mono-2-ethylhexyl phthalate hydrolase belonging to the serine hydrolase family - Iwata_2016_J.Biosci.Bioeng_122_140 |
| Author(s) : Iwata M , Imaoka T , Nishiyama T , Fujii T |
| Ref : J Biosci Bioeng , 122 :140 , 2016 |
| Abstract : Iwata_2016_J.Biosci.Bioeng_122_140 |
| ESTHER : Iwata_2016_J.Biosci.Bioeng_122_140 |
| PubMedSearch : Iwata_2016_J.Biosci.Bioeng_122_140 |
| PubMedID: 26868518 |
| Gene_locus related to this paper: 9noca-a0a0u5adh4 |