Ketoprofen-Ethyl-Ester
General
Type : Profen prodrug || Pro-Drug || Drug || Aryl ester || Propionate
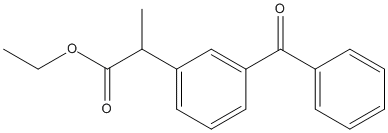
Chemical_Nomenclature : ethyl 2-(3-benzoylphenyl)propanoate
Canonical SMILES : CCOC(=O)C(C)C1=CC=CC(=C1)C(=O)C2=CC=CC=C2
InChI : InChI=1S\/C18H18O3\/c1-3-21-18(20)13(2)15-10-7-11-16(12-15)17(19)14-8-5-4-6-9-14\/h4-13H,3H2,1-2H3 || InChI=1S\/C18H18O3\/c1-3-21-18(20)13(2)15-10-7-11-16(12-15)17(19)14-8-5-4-6-9-14\/h4-13H,3H2,1-2H3\/t13-\/m0\/s1 || InChI=1S\/C18H18O3\/c1-3-21-18(20)13(2)15-10-7-11-16(12-15)17(19)14-8-5-4-6-9-14\/h4-13H,3H2,1-2H3\/t13-\/m1\/s1
InChIKey : CQSMNXCTDMLMLM-UHFFFAOYSA-N || CQSMNXCTDMLMLM-ZDUSSCGKSA-N || CQSMNXCTDMLMLM-CYBMUJFWSA-N
Other name(s) : Ketoprofen Ethyl Ester, Ethyl 2-(3-benzoylphenyl)propanoate, Ketoprofen ethyl, SCHEMBL9555892, DTXSID00432246, (S)-Ketoprofen ethyl ester, Dexketoprofen Ethyl Ester, ZINC34003604, Ethyl (2S)-2-(3-benzoylphenyl)propanoate, (R)-Ketoprofenethylester, (r)-ketoprofen ethyl ester, ZINC34003605
Target
References (11)
| Title : Enhanced Production of (S)-2-arylpropionic Acids by Protein Engineering and Whole-Cell Catalysis - Liu_2021_Front.Bioeng.Biotechnol_9_697677 |
| Author(s) : Liu X , Zhao M , Fan X , Fu Y |
| Ref : Front Bioeng Biotechnol , 9 :697677 , 2021 |
| Abstract : Liu_2021_Front.Bioeng.Biotechnol_9_697677 |
| ESTHER : Liu_2021_Front.Bioeng.Biotechnol_9_697677 |
| PubMedSearch : Liu_2021_Front.Bioeng.Biotechnol_9_697677 |
| PubMedID: 34307324 |
| Gene_locus related to this paper: 9zzzz-Est924 |
| Title : Crystal structure and characterization of esterase Est25 mutants reveal improved enantioselectivity toward (S)-ketoprofen ethyl ester - Kim_2017_Appl.Microbiol.Biotechnol_101_2333 |
| Author(s) : Kim J , Seok SH , Hong E , Yoo TH , Seo MD , Ryu Y |
| Ref : Applied Microbiology & Biotechnology , 101 :2333 , 2017 |
| Abstract : Kim_2017_Appl.Microbiol.Biotechnol_101_2333 |
| ESTHER : Kim_2017_Appl.Microbiol.Biotechnol_101_2333 |
| PubMedSearch : Kim_2017_Appl.Microbiol.Biotechnol_101_2333 |
| PubMedID: 27915377 |
| Gene_locus related to this paper: 9bact-q4tzq3 |
| Title : Improved enantioselectivity of thermostable esterase from Archaeoglobus fulgidus toward (S)-ketoprofen ethyl ester by directed evolution and characterization of mutant esterases - Kim_2015_Appl.Microbiol.Biotechnol_99_6293 |
| Author(s) : Kim J , Kim S , Yoon S , Hong E , Ryu Y |
| Ref : Applied Microbiology & Biotechnology , 99 :6293 , 2015 |
| Abstract : Kim_2015_Appl.Microbiol.Biotechnol_99_6293 |
| ESTHER : Kim_2015_Appl.Microbiol.Biotechnol_99_6293 |
| PubMedSearch : Kim_2015_Appl.Microbiol.Biotechnol_99_6293 |
| PubMedID: 25661815 |
| Title : Understanding the plasticity of the alpha\/beta hydrolase fold: lid swapping on the Candida antarctica lipase B results in chimeras with interesting biocatalytic properties - Skjot_2009_Chembiochem_10_520 |
| Author(s) : Skjot M , De Maria L , Chatterjee R , Svendsen A , Patkar SA , Ostergaard PR , Brask J |
| Ref : Chembiochem , 10 :520 , 2009 |
| Abstract : Skjot_2009_Chembiochem_10_520 |
| ESTHER : Skjot_2009_Chembiochem_10_520 |
| PubMedSearch : Skjot_2009_Chembiochem_10_520 |
| PubMedID: 19156649 |
| Gene_locus related to this paper: canar-LipB |
| Title : Purification, crystallization and preliminary crystallographic analysis of Est25: a ketoprofen-specific hormone-sensitive lipase - Kim_2007_Acta.Crystallogr.Sect.F.Struct.Biol.Cryst.Commun_63_579 |
| Author(s) : Kim S , Joo S , Yoon HC , Ryu Y , Kim KK , Kim TD |
| Ref : Acta Crystallographica Sect F Struct Biol Cryst Commun , 63 :579 , 2007 |
| Abstract : Kim_2007_Acta.Crystallogr.Sect.F.Struct.Biol.Cryst.Commun_63_579 |
| ESTHER : Kim_2007_Acta.Crystallogr.Sect.F.Struct.Biol.Cryst.Commun_63_579 |
| PubMedSearch : Kim_2007_Acta.Crystallogr.Sect.F.Struct.Biol.Cryst.Commun_63_579 |
| PubMedID: 17620715 |
| Gene_locus related to this paper: 9bact-q4tzq3 |
| Title : Overexpression of Serratia marcescens lipase in Escherichia coli for efficient bioresolution of racemic ketoprofen - Long_2007_J.Mol.Catal.B.Enzym_47_105 |
| Author(s) : Long ZD , Xu JH , Zhao LL , Pan J , Yang S , Hua L |
| Ref : J Mol Catal B Enzym , 47 :105 , 2007 |
| Abstract : Long_2007_J.Mol.Catal.B.Enzym_47_105 |
| ESTHER : Long_2007_J.Mol.Catal.B.Enzym_47_105 |
| PubMedSearch : Long_2007_J.Mol.Catal.B.Enzym_47_105 |
| PubMedID: |
| Gene_locus related to this paper: serma-lipasA |
| Title : Isolation and characterization of Acinetobacter sp. ES-1 excreting a lipase with high enantioselectivity for (S)-ketoprofen ethyl ester - Lee_2004_Biotechnol.Lett_26_1639 |
| Author(s) : Lee KW , Shin GS , Bae HA , Shin HD , Lee YH |
| Ref : Biotechnol Lett , 26 :1639 , 2004 |
| Abstract : Lee_2004_Biotechnol.Lett_26_1639 |
| ESTHER : Lee_2004_Biotechnol.Lett_26_1639 |
| PubMedSearch : Lee_2004_Biotechnol.Lett_26_1639 |
| PubMedID: 15604812 |
| Title : Integration of purification with immobilization of Candida rugosa lipase for kinetic resolution of racemic ketoprofen - Liu_2004_J.Biotechnol_110_209 |
| Author(s) : Liu YY , Xu JH , Wu HY , Shen D |
| Ref : J Biotechnol , 110 :209 , 2004 |
| Abstract : Liu_2004_J.Biotechnol_110_209 |
| ESTHER : Liu_2004_J.Biotechnol_110_209 |
| PubMedSearch : Liu_2004_J.Biotechnol_110_209 |
| PubMedID: 15121339 |
| Title : Kinetic resolution of ketoprofen ester catalyzed by lipase from a mutant of CBS 5791 - Liu_2004_J.Ind.Microbiol.Biotechnol_31_495 |
| Author(s) : Liu J , Zhang Y , Qiu L , Yang F , Ye L , Xia Y |
| Ref : J Ind Microbiol Biotechnol , 31 :495 , 2004 |
| Abstract : Liu_2004_J.Ind.Microbiol.Biotechnol_31_495 |
| ESTHER : Liu_2004_J.Ind.Microbiol.Biotechnol_31_495 |
| PubMedSearch : Liu_2004_J.Ind.Microbiol.Biotechnol_31_495 |
| PubMedID: 15538656 |
| Title : Construction and characterization of a recombinant esterase with high activity and enantioselectivity to (S)-ketoprofen ethyl ester - Choi_2003_Protein.Expr.Purif_29_85 |
| Author(s) : Choi GS , Kim JY , Kim JH , Ryu YW , Kim GJ |
| Ref : Protein Expr Purif , 29 :85 , 2003 |
| Abstract : Choi_2003_Protein.Expr.Purif_29_85 |
| ESTHER : Choi_2003_Protein.Expr.Purif_29_85 |
| PubMedSearch : Choi_2003_Protein.Expr.Purif_29_85 |
| PubMedID: 12729729 |
| Title : Production of optically active ketoprofen by direct enzymatic esterification - Park_1999_J.Biosci.Bioeng_87_545 |
| Author(s) : Park HJ , Choi WJ , Huh EC , Lee EY , Choi CY |
| Ref : J Biosci Bioeng , 87 :545 , 1999 |
| Abstract : Park_1999_J.Biosci.Bioeng_87_545 |
| ESTHER : Park_1999_J.Biosci.Bioeng_87_545 |
| PubMedSearch : Park_1999_J.Biosci.Bioeng_87_545 |
| PubMedID: 16232514 |