Juvenile-hormone-III
General
Type : Natural || Lactone || Epoxide
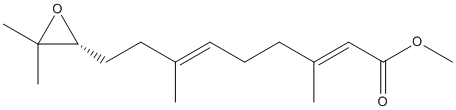
Chemical_Nomenclature : methyl (2E,6E)-9-[(2R)-3,3-dimethyloxiran-2-yl]-3,7-dimethylnona-2,6-dienoate
Canonical SMILES : CC(=CCCC(=CC(=O)OC)C)CCC1C(O1)(C)C
InChI : InChI=1S\/C16H26O3\/c1-12(9-10-14-16(3,4)19-14)7-6-8-13(2)11-15(17)18-5\/h7,11,14H,6,8-10H2,1-5H3\/b12-7+,13-11+\/t14-\/m1\/s1
InChIKey : QVJMXSGZTCGLHZ-HONBPKQLSA-N
Other name(s) : Juvenile hormone III, JH III, JHIII, JH-III, Juvenile hormone 3, (+)-Juvenile hormone III, (10R)-Juvenile hormone III, Methyl R-(+)-10,11-epoxyfarnesate
MW : 266.4
Formula : C16H26O3
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Epoxide_hydrolase, Juvenile_hormone_esterase
References (8)
| Title : Juvenile Hormone Is an Important Factor in Regulating Aspongopus chinensis Dallas Diapause - Zhou_2022_Front.Physiol_13_873580 |
| Author(s) : Zhou WZ , Wu YF , Yin ZY , Guo JJ , Li HY |
| Ref : Front Physiol , 13 :873580 , 2022 |
| Abstract : Zhou_2022_Front.Physiol_13_873580 |
| ESTHER : Zhou_2022_Front.Physiol_13_873580 |
| PubMedSearch : Zhou_2022_Front.Physiol_13_873580 |
| PubMedID: 35615668 |
| Title : Substrate and inhibitor selectivity, and biological activity of an epoxide hydrolase from Trichoderma reesei - de Oliveira_2019_Mol.Biol.Rep_46_371 |
| Author(s) : de Oliveira GS , Adriani PP , Wu H , Morisseau C , Hammock BD , Chambergo FS |
| Ref : Mol Biol Rep , 46 :371 , 2019 |
| Abstract : de Oliveira_2019_Mol.Biol.Rep_46_371 |
| ESTHER : de Oliveira_2019_Mol.Biol.Rep_46_371 |
| PubMedSearch : de Oliveira_2019_Mol.Biol.Rep_46_371 |
| PubMedID: 30426381 |
| Gene_locus related to this paper: hypjq-g0r7e2 |
| Title : Expression and characterization of an epoxide hydrolase from Anopheles gambiae with high activity on epoxy fatty acids - Xu_2014_Insect.Biochem.Mol.Biol_54C_42 |
| Author(s) : Xu J , Morisseau C , Hammock BD |
| Ref : Insect Biochemistry & Molecular Biology , 54C :42 , 2014 |
| Abstract : Xu_2014_Insect.Biochem.Mol.Biol_54C_42 |
| ESTHER : Xu_2014_Insect.Biochem.Mol.Biol_54C_42 |
| PubMedSearch : Xu_2014_Insect.Biochem.Mol.Biol_54C_42 |
| PubMedID: 25173592 |
| Title : Characterization and cDNA cloning of a clofibrate-inducible microsomal epoxide hydrolase in Drosophila melanogaster - Taniai_2003_Eur.J.Biochem_270_4696 |
| Author(s) : Taniai K , Inceoglu AB , Yukuhiro K , Hammock BD |
| Ref : European Journal of Biochemistry , 270 :4696 , 2003 |
| Abstract : Taniai_2003_Eur.J.Biochem_270_4696 |
| ESTHER : Taniai_2003_Eur.J.Biochem_270_4696 |
| PubMedSearch : Taniai_2003_Eur.J.Biochem_270_4696 |
| PubMedID: 14622257 |
| Gene_locus related to this paper: drome-CG15102 |
| Title : Cloning, partial purification and in vivo developmental profile of expression of the juvenile hormone epoxide hydrolase of Ctenocephalides felis - Keiser_2002_Arch.Insect.Biochem.Physiol_50_191 |
| Author(s) : Keiser KC , Brandt KS , Silver GM , Wisnewski N |
| Ref : Archives of Insect Biochemistry & Physiology , 50 :191 , 2002 |
| Abstract : Keiser_2002_Arch.Insect.Biochem.Physiol_50_191 |
| ESTHER : Keiser_2002_Arch.Insect.Biochem.Physiol_50_191 |
| PubMedSearch : Keiser_2002_Arch.Insect.Biochem.Physiol_50_191 |
| PubMedID: 12125060 |
| Gene_locus related to this paper: ctefe-Q8MZR5 , ctefe-Q8MZR6 |
| Title : Cloning and expression of a novel juvenile hormone-metabolizing epoxide hydrolase during larval-pupal metamorphosis of the cabbage looper, Trichoplusia ni - VanHook_1999_Insect.Mol.Biol_8_85 |
| Author(s) : VanHook Harris S , Marin Thompson D , Linderman RJ , Tomalski MD , Roe RM |
| Ref : Insect Molecular Biology , 8 :85 , 1999 |
| Abstract : VanHook_1999_Insect.Mol.Biol_8_85 |
| ESTHER : VanHook_1999_Insect.Mol.Biol_8_85 |
| PubMedSearch : VanHook_1999_Insect.Mol.Biol_8_85 |
| PubMedID: 9927177 |
| Gene_locus related to this paper: trini-Q94806 |
| Title : Purification and kinetic characterisation of juvenile hormone esterase from Drosophila melanogaster - Campbell_1998_Insect.Biochem.Mol.Biol_28_501 |
| Author(s) : Campbell PM , Oakeshott JG , Healy MJ |
| Ref : Insect Biochemistry & Molecular Biology , 28 :501 , 1998 |
| Abstract : Campbell_1998_Insect.Biochem.Mol.Biol_28_501 |
| ESTHER : Campbell_1998_Insect.Biochem.Mol.Biol_28_501 |
| PubMedSearch : Campbell_1998_Insect.Biochem.Mol.Biol_28_501 |
| PubMedID: 9718682 |
| Gene_locus related to this paper: drome-CG8425 |
| Title : Molecular and biochemical evidence for the involvement of the Asp-333- His-523 pair in the catalytic mechanism of soluble epoxide hydrolase - Pinot_1995_J.Biol.Chem_270_7968 |
| Author(s) : Pinot F , Grant DF , Beetham JK , Parker AG , Borhan B , Landt S , Jones AD , Hammock BD |
| Ref : Journal of Biological Chemistry , 270 :7968 , 1995 |
| Abstract : Pinot_1995_J.Biol.Chem_270_7968 |
| ESTHER : Pinot_1995_J.Biol.Chem_270_7968 |
| PubMedSearch : Pinot_1995_J.Biol.Chem_270_7968 |
| PubMedID: 7713895 |