Juvenile-hormone-II
General
Type : Natural || Lactone || Epoxide
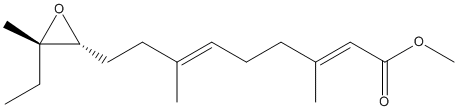
Chemical_Nomenclature : methyl (2E,6E)-9-[(2R,3S)-3-ethyl-3-methyloxiran-2-yl]-3,7-dimethylnona-2,6-dienoate
Canonical SMILES : CCC1(C(O1)CCC(=CCCC(=CC(=O)OC)C)C)C
InChI : InChI=1S\/C17H28O3\/c1-6-17(4)15(20-17)11-10-13(2)8-7-9-14(3)12-16(18)19-5\/h8,12,15H,6-7,9-11H2,1-5H3\/b13-8+,14-12+\/t15-,17+\/m1\/s1
InChIKey : CPVQJXZBSGXTGJ-TZDLBHCHSA-N
Other name(s) : Juvenile hormone II, JH-II, JH II, JHII, 12-Homojuvenate, Juvenile hormone 2
Target
Families : Epoxide_hydrolase
References (4)
| Title : Crystal structure of juvenile hormone epoxide hydrolase from the silkworm Bombyx mori - Zhou_2014_Proteins_82_3224 |
| Author(s) : Zhou K , Jia N , Hu C , Jiang YL , Yang JP , Chen Y , Li S , Li WF , Zhou CZ |
| Ref : Proteins , 82 :3224 , 2014 |
| Abstract : Zhou_2014_Proteins_82_3224 |
| ESTHER : Zhou_2014_Proteins_82_3224 |
| PubMedSearch : Zhou_2014_Proteins_82_3224 |
| PubMedID: 25143157 |
| Gene_locus related to this paper: bommo-q6u6j0 |
| Title : The ectoparasitic wasp Eulophus pennicornis (Hymenoptera: Eulophidae) uses instar-specific endocrine disruption strategies to suppress the development of its host Lacanobia oleracea (Lepidoptera: Noctuidae) - Edwards_2006_J.Insect.Physiol_52_1153 |
| Author(s) : Edwards JP , Bell HA , Audsley N , Marris GC , Kirkbride-Smith A , Bryning G , Frisco C , Cusson M |
| Ref : J Insect Physiol , 52 :1153 , 2006 |
| Abstract : Edwards_2006_J.Insect.Physiol_52_1153 |
| ESTHER : Edwards_2006_J.Insect.Physiol_52_1153 |
| PubMedSearch : Edwards_2006_J.Insect.Physiol_52_1153 |
| PubMedID: 17064726 |
| Title : Juvenile hormone catabolism and oviposition in the codling moth, Cydia pomonella, as functions of age, mating status, and hormone treatment - Cole_2002_Arch.Insect.Biochem.Physiol_49_10 |
| Author(s) : Cole TJ , Ramaswamy SB , Srinivasan A , Dorn S |
| Ref : Archives of Insect Biochemistry & Physiology , 49 :10 , 2002 |
| Abstract : Cole_2002_Arch.Insect.Biochem.Physiol_49_10 |
| ESTHER : Cole_2002_Arch.Insect.Biochem.Physiol_49_10 |
| PubMedSearch : Cole_2002_Arch.Insect.Biochem.Physiol_49_10 |
| PubMedID: 11754090 |
| Title : Characterization of affinity-purified juvenile hormone esterase from Trichoplusia ni - Hanzlik_1987_J.Biol.Chem_262_13584 |
| Author(s) : Hanzlik TN , Hammock BD |
| Ref : Journal of Biological Chemistry , 262 :13584 , 1987 |
| Abstract : Hanzlik_1987_J.Biol.Chem_262_13584 |
| ESTHER : Hanzlik_1987_J.Biol.Chem_262_13584 |
| PubMedSearch : Hanzlik_1987_J.Biol.Chem_262_13584 |
| PubMedID: 3654630 |
| Gene_locus related to this paper: trini-jhestrp |