Isobutyl-cinnamate
General
Type : Aryl ester
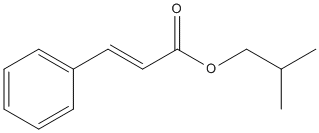
Chemical_Nomenclature : 2-methylpropyl (E)-3-phenylprop-2-enoate
Canonical SMILES : CC(C)COC(=O)C=CC1=CC=CC=C1
InChI : InChI=1S\/C13H16O2\/c1-11(2)10-15-13(14)9-8-12-6-4-3-5-7-12\/h3-9,11H,10H2,1-2H3\/b9-8+
InChIKey : IQZUZPKOFSOVET-CMDGGOBGSA-N
Other name(s) : Isobutyl cinnamate, Labdanol, 2-Methylpropyl cinnamate, Cinnamic acid, isobutyl ester, 2-Methylpropyl 3-phenylpropenoate
Target
Families : No family
References (3)
| Title : Lipase-Catalyzed Production of 6-O-cinnamoyl-sorbitol from D-sorbitol and Cinnamic Acid Esters - Kim_2015_Appl.Biochem.Biotechnol_176_244 |
| Author(s) : Kim JH , Bhatia SK , Yoo D , Seo HM , Yi DH , Kim HJ , Lee JH , Choi KY , Kim KJ , Lee YK , Yang YH |
| Ref : Appl Biochem Biotechnol , 176 :244 , 2015 |
| Abstract : Kim_2015_Appl.Biochem.Biotechnol_176_244 |
| ESTHER : Kim_2015_Appl.Biochem.Biotechnol_176_244 |
| PubMedSearch : Kim_2015_Appl.Biochem.Biotechnol_176_244 |
| PubMedID: 25809993 |
| Title : Fragrance material review on isobutyl cinnamate - Bhatia_2007_Food.Chem.Toxicol_45 Suppl 1_S102 |
| Author(s) : Bhatia SP , Wellington GA , Cocchiara J , Lalko J , Letizia CS , Api AM |
| Ref : Food & Chemical Toxicology , 45 Suppl 1 :S102 , 2007 |
| Abstract : Bhatia_2007_Food.Chem.Toxicol_45 Suppl 1_S102 |
| ESTHER : Bhatia_2007_Food.Chem.Toxicol_45 Suppl 1_S102 |
| PubMedSearch : Bhatia_2007_Food.Chem.Toxicol_45 Suppl 1_S102 |
| PubMedID: 18036714 |