Heroin
General
Type : Natural || Drug || Isoquinoline || Alkaloid
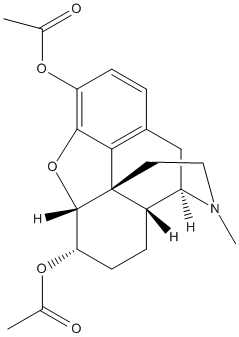
Chemical_Nomenclature : [(4R,4aR,7S,7aR,12bS)-9-acetyloxy-3-methyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7-yl] acetate
Canonical SMILES : CC(=O)OC1C=CC2C3CC4=C5C2(C1OC5=C(C=C4)OC(=O)C)CCN3C
InChI : InChI=1S\/C21H23NO5\/c1-11(23)25-16-6-4-13-10-15-14-5-7-17(26-12(2)24)20-21(14,8-9-22(15)3)18(13)19(16)27-20\/h4-7,14-15,17,20H,8-10H2,1-3H3\/t14-,15+,17-,20-,21-\/m0\/s1
InChIKey : GVGLGOZIDCSQPN-PVHGPHFFSA-N
Other name(s) : Diacetylmorphine, 7,8-Didehydro-4,5alpha-epoxy-17-methylmorphinane-3,6alpha-diol diacetate (ester), Diamorphine, 3,6-Diacetylmorphine, Acetomorphine, Diaphorm
Target
Families : BCHE, Carb_B_Chordata
References (5)
| Title : Fundamental reaction pathway and free energy profile for butyrylcholinesterase-catalyzed hydrolysis of heroin - Qiao_2013_Biochemistry_52_6467 |
| Author(s) : Qiao Y , Han K , Zhan CG |
| Ref : Biochemistry , 52 :6467 , 2013 |
| Abstract : Qiao_2013_Biochemistry_52_6467 |
| ESTHER : Qiao_2013_Biochemistry_52_6467 |
| PubMedSearch : Qiao_2013_Biochemistry_52_6467 |
| PubMedID: 23992153 |
| Title : Biochemical and molecular analysis of carboxylesterase-mediated hydrolysis of cocaine and heroin - Hatfield_2010_Br.J.Pharmacol_160_1916 |
| Author(s) : Hatfield MJ , Tsurkan LG , Hyatt JL , Yu X , Edwards CC , Hicks LD , Wadkins RM , Potter PM |
| Ref : British Journal of Pharmacology , 160 :1916 , 2010 |
| Abstract : Hatfield_2010_Br.J.Pharmacol_160_1916 |
| ESTHER : Hatfield_2010_Br.J.Pharmacol_160_1916 |
| PubMedSearch : Hatfield_2010_Br.J.Pharmacol_160_1916 |
| PubMedID: 20649590 |
| Gene_locus related to this paper: human-CES1 |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : Alteration of in vivo and in vitro effects of heroin by esterase inhibition - Gianutsos_1986_Toxicol.Appl.Pharmacol_82_14 |
| Author(s) : Gianutsos G , Cohen SD , Carlson G , Heyman R , Salva P , Morrow G , Hite GJ |
| Ref : Toxicol Appl Pharmacol , 82 :14 , 1986 |
| Abstract : Gianutsos_1986_Toxicol.Appl.Pharmacol_82_14 |
| ESTHER : Gianutsos_1986_Toxicol.Appl.Pharmacol_82_14 |
| PubMedSearch : Gianutsos_1986_Toxicol.Appl.Pharmacol_82_14 |
| PubMedID: 3003965 |
| Title : Hydrolysis of diacetylmorphine (heroin) by human serum cholinesterase - Lockridge_1980_J.Pharmacol.Exp.Ther_215_1 |
| Author(s) : Lockridge O , Mottershaw-Jackson N , Eckerson HW , La Du BN |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 215 :1 , 1980 |
| Abstract : Lockridge_1980_J.Pharmacol.Exp.Ther_215_1 |
| ESTHER : Lockridge_1980_J.Pharmacol.Exp.Ther_215_1 |
| PubMedSearch : Lockridge_1980_J.Pharmacol.Exp.Ther_215_1 |
| PubMedID: 7452476 |