HOPDA-5,8-diF
General
Type : Ketone
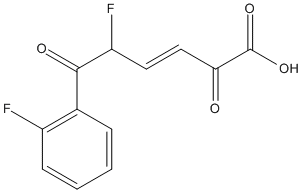
Chemical_Nomenclature : (3E,5R)-5-fluoro-6-(2-fluorophenyl)-2,6-dioxohex-3-enoic acid
Canonical SMILES : OC(=O)C(=O)C=CC(F)C(=O)c1ccccc1F
InChI : InChI=1S\/C12H8F2O4\/c13-8-4-2-1-3-7(8)11(16)9(14)5-6-10(15)12(17)18\/h1-6,9H,(H,17,18)\/b6-5+\/t9-\/m1\/s1
InChIKey : YTBJKOAMJGQNPQ-VUHVRTRXSA-N
Other name(s) : (3E,5R)-5-Fluoro-6-(2-fluorophenyl)-2,6-dioxo-3-hexenoic acid, 22J
MW : 254.18
Formula : C12H8F2O4
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Carbon-carbon_bond_hydrolase
References (2)
| Title : A substrate-assisted mechanism of nucleophile activation in a ser-his-asp containing C-C bond hydrolase - Ruzzini_2013_Biochemistry_52_7428 |
| Author(s) : Ruzzini AC , Bhowmik S , Ghosh S , Yam KC , Bolin JT , Eltis LD |
| Ref : Biochemistry , 52 :7428 , 2013 |
| Abstract : Ruzzini_2013_Biochemistry_52_7428 |
| ESTHER : Ruzzini_2013_Biochemistry_52_7428 |
| PubMedSearch : Ruzzini_2013_Biochemistry_52_7428 |
| PubMedID: 24067021 |
| Gene_locus related to this paper: sphww-a5j2a5 |
| Title : The Lid Domain of the MCP Hydrolase DxnB2 Contributes to the Reactivity toward Recalcitrant PCB Metabolites - Ruzzini_2013_Biochemistry_52_5685 |
| Author(s) : Ruzzini AC , Bhowmik S , Yam KC , Ghosh S , Bolin JT , Eltis LD |
| Ref : Biochemistry , 52 :5685 , 2013 |
| Abstract : Ruzzini_2013_Biochemistry_52_5685 |
| ESTHER : Ruzzini_2013_Biochemistry_52_5685 |
| PubMedSearch : Ruzzini_2013_Biochemistry_52_5685 |
| PubMedID: 23879719 |
| Gene_locus related to this paper: sphww-a5j2a5 |