HEPTAT
General
Type : Sulfur compound
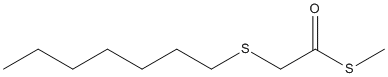
Chemical_Nomenclature : S-methyl 2-heptylsulfanylethanethioate
Canonical SMILES : CCCCCCCSCC(=O)SC
InChI : InChI=1S\/C10H20OS2\/c1-3-4-5-6-7-8-13-9-10(11)12-2\/h3-9H2,1-2H3
InChIKey : DOIXAEGXYWJNKL-UHFFFAOYSA-N
Other name(s) : Ethanethioic acid, (heptylthio)-, S-methyl ester, ACMC-20n6q1, CTK0B1159, DTXSID30772581, S-Methyl (heptylsulfanyl)ethanethioate
Target
Families : Juvenile_hormone_esterase
References (10)
| Title : Juvenile hormone (JH) esterase of the mosquito Culex quinquefasciatus is not a target of the JH analog insecticide methoprene - Kamita_2011_PLoS.One_6_e28392 |
| Author(s) : Kamita SG , Samra AI , Liu JY , Cornel AJ , Hammock BD |
| Ref : PLoS ONE , 6 :e28392 , 2011 |
| Abstract : Kamita_2011_PLoS.One_6_e28392 |
| ESTHER : Kamita_2011_PLoS.One_6_e28392 |
| PubMedSearch : Kamita_2011_PLoS.One_6_e28392 |
| PubMedID: 22174797 |
| Gene_locus related to this paper: culqu-b0w3q7 |
| Title : Function of phenylalanine 259 and threonine 314 within the substrate binding pocket of the juvenile hormone esterase of Manduca sexta - Kamita_2010_Biochemistry_49_3733 |
| Author(s) : Kamita SG , Wogulis MD , Law CS , Morisseau C , Tanaka H , Huang H , Wilson DK , Hammock BD |
| Ref : Biochemistry , 49 :3733 , 2010 |
| Abstract : Kamita_2010_Biochemistry_49_3733 |
| ESTHER : Kamita_2010_Biochemistry_49_3733 |
| PubMedSearch : Kamita_2010_Biochemistry_49_3733 |
| PubMedID: 20307057 |
| Title : Juvenile hormone esterase: biochemistry and structure - Kamita_2010_J.Pestic.Sci_35_265 |
| Author(s) : Kamita SG , Hammock BD |
| Ref : Journal of Pesticide Science , 35 :265 , 2010 |
| Abstract : Kamita_2010_J.Pestic.Sci_35_265 |
| ESTHER : Kamita_2010_J.Pestic.Sci_35_265 |
| PubMedSearch : Kamita_2010_J.Pestic.Sci_35_265 |
| PubMedID: 23543805 |
| Title : Structural studies of a potent insect maturation inhibitor bound to the juvenile hormone esterase of Manduca sexta - Wogulis_2006_Biochemistry_45_4045 |
| Author(s) : Wogulis M , Wheelock CE , Kamita SG , Hinton AC , Whetstone PA , Hammock BD , Wilson DK |
| Ref : Biochemistry , 45 :4045 , 2006 |
| Abstract : Wogulis_2006_Biochemistry_45_4045 |
| ESTHER : Wogulis_2006_Biochemistry_45_4045 |
| PubMedSearch : Wogulis_2006_Biochemistry_45_4045 |
| PubMedID: 16566578 |
| Gene_locus related to this paper: manse-jhest |
| Title : Juvenile hormone esterase (JHE) from Tenebrio molitor: full-length cDNA sequence, in vitro expression, and characterization of the recombinant protein - Hinton_2003_Insect.Biochem.Mol.Biol_33_477 |
| Author(s) : Hinton AC , Hammock BD |
| Ref : Insect Biochemistry & Molecular Biology , 33 :477 , 2003 |
| Abstract : Hinton_2003_Insect.Biochem.Mol.Biol_33_477 |
| ESTHER : Hinton_2003_Insect.Biochem.Mol.Biol_33_477 |
| PubMedSearch : Hinton_2003_Insect.Biochem.Mol.Biol_33_477 |
| PubMedID: 12706627 |
| Gene_locus related to this paper: tenmo-Q8WRD8 |
| Title : In vitro expression and biochemical characterization of juvenile hormone esterase from Manduca sexta - Hinton_2003_Insect.Biochem.Mol.Biol_33_317 |
| Author(s) : Hinton AC , Hammock BD |
| Ref : Insect Biochemistry & Molecular Biology , 33 :317 , 2003 |
| Abstract : Hinton_2003_Insect.Biochem.Mol.Biol_33_317 |
| ESTHER : Hinton_2003_Insect.Biochem.Mol.Biol_33_317 |
| PubMedSearch : Hinton_2003_Insect.Biochem.Mol.Biol_33_317 |
| PubMedID: 12609517 |
| Title : cDNA cloning and characterization of Bombyx mori juvenile hormone esterase: an inducible gene by the imidazole insect growth regulator KK-42 - Hirai_2002_Insect.Biochem.Mol.Biol_32_627 |
| Author(s) : Hirai M , Kamimura M , Kikuchi K , Yasukochi Y , Kiuchi M , Shinoda T , Shiotsuki T |
| Ref : Insect Biochemistry & Molecular Biology , 32 :627 , 2002 |
| Abstract : Hirai_2002_Insect.Biochem.Mol.Biol_32_627 |
| ESTHER : Hirai_2002_Insect.Biochem.Mol.Biol_32_627 |
| PubMedSearch : Hirai_2002_Insect.Biochem.Mol.Biol_32_627 |
| PubMedID: 12020837 |
| Gene_locus related to this paper: bommo-JHE |
| Title : Characterization and affinity purification of juvenile hormone esterase from Bombyx mori - Shiotsuki_2000_Biosci.Biotechnol.Biochem_64_1681 |
| Author(s) : Shiotsuki T , Bonning BC , Hirai M , Kikuchi K , Hammock BD |
| Ref : Biosci Biotechnol Biochem , 64 :1681 , 2000 |
| Abstract : Shiotsuki_2000_Biosci.Biotechnol.Biochem_64_1681 |
| ESTHER : Shiotsuki_2000_Biosci.Biotechnol.Biochem_64_1681 |
| PubMedSearch : Shiotsuki_2000_Biosci.Biotechnol.Biochem_64_1681 |
| PubMedID: 10993156 |
| Title : Disruption of lysosomal targeting is associated with insecticidal potency of juvenile hormone esterase - Bonning_1997_Proc.Natl.Acad.Sci.U.S.A_94_6007 |
| Author(s) : Bonning BC , Ward VK , van Meer MM , Booth TF , Hammock BD |
| Ref : Proc Natl Acad Sci U S A , 94 :6007 , 1997 |
| Abstract : Bonning_1997_Proc.Natl.Acad.Sci.U.S.A_94_6007 |
| ESTHER : Bonning_1997_Proc.Natl.Acad.Sci.U.S.A_94_6007 |
| PubMedSearch : Bonning_1997_Proc.Natl.Acad.Sci.U.S.A_94_6007 |
| PubMedID: 9177159 |
| Title : Development of surrogate substrates for juvenile hormone esterase - McCutchen_1993_Arch.Biochem.Biophys_307_231 |
| Author(s) : McCutchen BF , Uematsu T , Szekacs A , Huang TL , Shiotsuki T , Lucas A , Hammock BD |
| Ref : Archives of Biochemistry & Biophysics , 307 :231 , 1993 |
| Abstract : McCutchen_1993_Arch.Biochem.Biophys_307_231 |
| ESTHER : McCutchen_1993_Arch.Biochem.Biophys_307_231 |
| PubMedSearch : McCutchen_1993_Arch.Biochem.Biophys_307_231 |
| PubMedID: 8274008 |