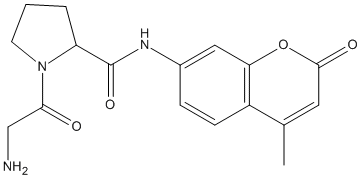
Gly-Pro-AMC
General
Type : Coumarin || Peptide || Pyrrolidine || Carboxamide || 7-amido-4-methylcoumarin
Chemical_Nomenclature : 1-(2-aminoacetyl)-N-(4-methyl-2-oxochromen-7-yl)pyrrolidine-2-carboxamide
Canonical SMILES : CC1=CC(=O)OC2=C1C=CC(=C2)NC(=O)C3CCCN3C(=O)CN
InChI : InChI=1S\/C17H19N3O4\/c1-10-7-16(22)24-14-8-11(4-5-12(10)14)19-17(23)13-3-2-6-20(13)15(21)9-18\/h4-5,7-8,13H,2-3,6,9,18H2,1H3,(H,19,23)
InChIKey : UTDVHCQTKWTQEA-UHFFFAOYSA-N
Other name(s) : Gly-Pro-7-amido-4-methylcoumarin hydrobromide, AC1N2LFZ, Gly-Pro-7-amidomethylcoumarin, AMC039, SCHEMBL11440079
MW : 329.35
Formula : C17H19N3O4
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : DPP4N_Peptidase_S9
References (3)
| Title : Outside or inside: role of the subcellular localization of DP4-like enzymes for substrate conversion and inhibitor effects - Bank_2011_Biol.Chem_392_169 |
| Author(s) : Bank U , Heimburg A , Wohlfarth A , Koch G , Nordhoff K , Julius H , Helmuth M , Breyer D , Reinhold D , Tager M , Ansorge S |
| Ref : Biol Chem , 392 :169 , 2011 |
| Abstract : Bank_2011_Biol.Chem_392_169 |
| ESTHER : Bank_2011_Biol.Chem_392_169 |
| PubMedSearch : Bank_2011_Biol.Chem_392_169 |
| PubMedID: 21194378 |
| Title : Nature of action of Sitagliptin, the dipeptidyl peptidase-IV inhibitor in diabetic animals - Davis_2010_Indian.J.Pharmacol_42_229 |
| Author(s) : Davis JA , Singh S , Sethi S , Roy S , Mittra S , Rayasam G , Bansal V , Sattigeri J , Ray A |
| Ref : Indian J Pharmacol , 42 :229 , 2010 |
| Abstract : Davis_2010_Indian.J.Pharmacol_42_229 |
| ESTHER : Davis_2010_Indian.J.Pharmacol_42_229 |
| PubMedSearch : Davis_2010_Indian.J.Pharmacol_42_229 |
| PubMedID: 20927248 |
| Title : NVP-DPP728 (1-[[[2-[(5-cyanopyridin-2-yl)amino]ethyl]amino]acetyl]-2-cyano-(S)- pyrrolidine), a slow-binding inhibitor of dipeptidyl peptidase IV - Hughes_1999_Biochemistry_38_11597 |
| Author(s) : Hughes TE , Mone MD , Russell ME , Weldon SC , Villhauer EB |
| Ref : Biochemistry , 38 :11597 , 1999 |
| Abstract : Hughes_1999_Biochemistry_38_11597 |
| ESTHER : Hughes_1999_Biochemistry_38_11597 |
| PubMedSearch : Hughes_1999_Biochemistry_38_11597 |
| PubMedID: 10512614 |