Gibberellin-A3
General
Type : Natural || Plant hormone
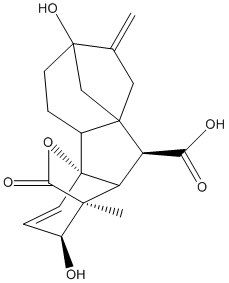
Chemical_Nomenclature : (1R,2R,5S,8S,9S,10R,11S,12S)-5,12-dihydroxy-11-methyl-6-methylidene-16-oxo-15-oxapentacyclo[9.3.2.15,8.01,10.02,8]heptadec-13-ene-9-carboxylic acid
Canonical SMILES : CC12C(C=CC3(C1C(C45C3CCC(C4)(C(=C)C5)O)C(=O)O)OC2=O)O
InChI : InChI=1S\/C19H22O6\/c1-9-7-17-8-18(9,24)5-3-10(17)19-6-4-11(20)16(2,15(23)25-19)13(19)12(17)14(21)22\/h4,6,10-13,20,24H,1,3,5,7-8H2,2H3,(H,21,22)\/t10-,11+,12-,13-,16-,17+,18+,19-\/m1\/s1
InChIKey : IXORZMNAPKEEDV-OBDJNFEBSA-N
Other name(s) : GA3, Gibberellic acid, Gibberellin Acid, Gibberellin A3, 77-06-5, Gibberellin, Gibreskol, Brellin
MW : 346.37
Formula : C19H22O6
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Plant_carboxylesterase
References (13)
| Title : Largely additive effects of gibberellin and strigolactone on gene expression in Arabidopsis thaliana seedlings - Lantzouni_2017_Plant.J_92_924 |
| Author(s) : Lantzouni O , Klermund C , Schwechheimer C |
| Ref : Plant J , 92 :924 , 2017 |
| Abstract : Lantzouni_2017_Plant.J_92_924 |
| ESTHER : Lantzouni_2017_Plant.J_92_924 |
| PubMedSearch : Lantzouni_2017_Plant.J_92_924 |
| PubMedID: 28977719 |
| Title : Computational gibberellin-binding channel discovery unraveling the unexpected perception mechanism of hormone signal by gibberellin receptor - Hao_2013_J.Comput.Chem_34_2055 |
| Author(s) : Hao GF , Yang SG , Yang GF , Zhan CG |
| Ref : J Comput Chem , 34 :2055 , 2013 |
| Abstract : Hao_2013_J.Comput.Chem_34_2055 |
| ESTHER : Hao_2013_J.Comput.Chem_34_2055 |
| PubMedSearch : Hao_2013_J.Comput.Chem_34_2055 |
| PubMedID: 23765254 |
| Title : Molecular determinants that convert hormone sensitive lipase into gibberellin receptor - Hirano_2012_Protein.Pept.Lett_19_180 |
| Author(s) : Hirano K , Aya K , Matsuoka M , Ueguchi-Tanaka M |
| Ref : Protein Pept Lett , 19 :180 , 2012 |
| Abstract : Hirano_2012_Protein.Pept.Lett_19_180 |
| ESTHER : Hirano_2012_Protein.Pept.Lett_19_180 |
| PubMedSearch : Hirano_2012_Protein.Pept.Lett_19_180 |
| PubMedID: 21933120 |
| Gene_locus related to this paper: orysa-gid1 |
| Title : Members of the gibberellin receptor gene family GID1 (GIBBERELLIN INSENSITIVE DWARF1) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination - Voegele_2011_J.Exp.Bot_62_5131 |
| Author(s) : Voegele A , Linkies A , Muller K , Leubner-Metzger G |
| Ref : J Exp Bot , 62 :5131 , 2011 |
| Abstract : Voegele_2011_J.Exp.Bot_62_5131 |
| ESTHER : Voegele_2011_J.Exp.Bot_62_5131 |
| PubMedSearch : Voegele_2011_J.Exp.Bot_62_5131 |
| PubMedID: 21778177 |
| Gene_locus related to this paper: lepsv-e2j7j8 |
| Title : The perception of gibberellins: clues from receptor structure - Ueguchi-Tanaka_2010_Curr.Opin.Plant.Biol_13_503 |
| Author(s) : Ueguchi-Tanaka M , Matsuoka M |
| Ref : Curr Opin Plant Biol , 13 :503 , 2010 |
| Abstract : Ueguchi-Tanaka_2010_Curr.Opin.Plant.Biol_13_503 |
| ESTHER : Ueguchi-Tanaka_2010_Curr.Opin.Plant.Biol_13_503 |
| PubMedSearch : Ueguchi-Tanaka_2010_Curr.Opin.Plant.Biol_13_503 |
| PubMedID: 20851040 |
| Title : Differential expression and affinities of Arabidopsis gibberellin receptors can explain variation in phenotypes of multiple knock-out mutants - Suzuki_2009_Plant.J_60_48 |
| Author(s) : Suzuki H , Park SH , Okubo K , Kitamura J , Ueguchi-Tanaka M , Iuchi S , Katoh E , Kobayashi M , Yamaguchi I , Matsuoka M , Asami T , Nakajima M |
| Ref : Plant J , 60 :48 , 2009 |
| Abstract : Suzuki_2009_Plant.J_60_48 |
| ESTHER : Suzuki_2009_Plant.J_60_48 |
| PubMedSearch : Suzuki_2009_Plant.J_60_48 |
| PubMedID: 19500306 |
| Gene_locus related to this paper: arath-AT5G27320 , arath-GID1B |
| Title : Structural basis for gibberellin recognition by its receptor GID1 - Shimada_2008_Nature_456_520 |
| Author(s) : Shimada A , Ueguchi-Tanaka M , Nakatsu T , Nakajima M , Naoe Y , Ohmiya H , Kato H , Matsuoka M |
| Ref : Nature , 456 :520 , 2008 |
| Abstract : Shimada_2008_Nature_456_520 |
| ESTHER : Shimada_2008_Nature_456_520 |
| PubMedSearch : Shimada_2008_Nature_456_520 |
| PubMedID: 19037316 |
| Gene_locus related to this paper: orysa-gid1 |
| Title : Gibberellin-induced DELLA recognition by the gibberellin receptor GID1 - Murase_2008_Nature_456_459 |
| Author(s) : Murase K , Hirano Y , Sun TP , Hakoshima T |
| Ref : Nature , 456 :459 , 2008 |
| Abstract : Murase_2008_Nature_456_459 |
| ESTHER : Murase_2008_Nature_456_459 |
| PubMedSearch : Murase_2008_Nature_456_459 |
| PubMedID: 19037309 |
| Gene_locus related to this paper: arath-GID1B |
| Title : Multiple loss-of-function of Arabidopsis gibberellin receptor AtGID1s completely shuts down a gibberellin signal - Iuchi_2007_Plant.J_50_958 |
| Author(s) : Iuchi S , Suzuki H , Kim YC , Iuchi A , Kuromori T , Ueguchi-Tanaka M , Asami T , Yamaguchi I , Matsuoka M , Kobayashi M , Nakajima M |
| Ref : Plant J , 50 :958 , 2007 |
| Abstract : Iuchi_2007_Plant.J_50_958 |
| ESTHER : Iuchi_2007_Plant.J_50_958 |
| PubMedSearch : Iuchi_2007_Plant.J_50_958 |
| PubMedID: 17521411 |
| Gene_locus related to this paper: arath-AT5G27320 , arath-GID1B |
| Title : Step-by-step acquisition of the gibberellin-DELLA growth-regulatory mechanism during land-plant evolution - Yasumura_2007_Curr.Biol_17_1225 |
| Author(s) : Yasumura Y , Crumpton-Taylor M , Fuentes S , Harberd NP |
| Ref : Current Biology , 17 :1225 , 2007 |
| Abstract : Yasumura_2007_Curr.Biol_17_1225 |
| ESTHER : Yasumura_2007_Curr.Biol_17_1225 |
| PubMedSearch : Yasumura_2007_Curr.Biol_17_1225 |
| PubMedID: 17627823 |
| Title : Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis - Griffiths_2006_Plant.Cell_18_3399 |
| Author(s) : Griffiths J , Murase K , Rieu I , Zentella R , Zhang ZL , Powers SJ , Gong F , Phillips AL , Hedden P , Sun TP , Thomas SG |
| Ref : Plant Cell , 18 :3399 , 2006 |
| Abstract : Griffiths_2006_Plant.Cell_18_3399 |
| ESTHER : Griffiths_2006_Plant.Cell_18_3399 |
| PubMedSearch : Griffiths_2006_Plant.Cell_18_3399 |
| PubMedID: 17194763 |
| Gene_locus related to this paper: arath-AT5G27320 , arath-GID1B |
| Title : Identification and characterization of Arabidopsis gibberellin receptors - Nakajima_2006_Plant.J_46_880 |
| Author(s) : Nakajima M , Shimada A , Takashi Y , Kim YC , Park SH , Ueguchi-Tanaka M , Suzuki H , Katoh E , Iuchi S , Kobayashi M , Maeda T , Matsuoka M , Yamaguchi I |
| Ref : Plant J , 46 :880 , 2006 |
| Abstract : Nakajima_2006_Plant.J_46_880 |
| ESTHER : Nakajima_2006_Plant.J_46_880 |
| PubMedSearch : Nakajima_2006_Plant.J_46_880 |
| PubMedID: 16709201 |
| Gene_locus related to this paper: arath-AT5G27320 , arath-AT5G62180 , arath-GID1B |
| Title : GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin - Ueguchi-Tanaka_2005_Nature_437_693 |
| Author(s) : Ueguchi-Tanaka M , Ashikari M , Nakajima M , Itoh H , Katoh E , Kobayashi M , Chow TY , Hsing YI , Kitano H , Yamaguchi I , Matsuoka M |
| Ref : Nature , 437 :693 , 2005 |
| Abstract : Ueguchi-Tanaka_2005_Nature_437_693 |
| ESTHER : Ueguchi-Tanaka_2005_Nature_437_693 |
| PubMedSearch : Ueguchi-Tanaka_2005_Nature_437_693 |
| PubMedID: 16193045 |
| Gene_locus related to this paper: orysa-gid1 |