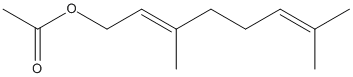
Geranyl-acetate
General
Type : Alkenyl ester || Acetate || Natural || Plant volatile
Chemical_Nomenclature : [(2E)-3,7-dimethylocta-2,6-dienyl] acetate
Canonical SMILES : CC(=CCCC(=CCOC(=O)C)C)C
InChI : InChI=1S\/C12H20O2\/c1-10(2)6-5-7-11(3)8-9-14-12(4)13\/h6,8H,5,7,9H2,1-4H3\/b11-8+
InChIKey : HIGQPQRQIQDZMP-DHZHZOJOSA-N
Other name(s) : Geranyl acetate, Geraniol acetate, Bay pine (oyster) oil, Acetic acid, geraniol ester, Trans-3,7-Dimethyl-2,6-octadien-1-yl acetate
Target
Families : Canar_LipB, Carb_B_Arthropoda
References (9)
| Title : An antenna-biased carboxylesterase is specifically active to plant volatiles in Spodoptera exigua - He_2015_Pestic.Biochem.Physiol_123_93 |
| Author(s) : He P , Zhang YN , Yang K , Li ZQ , Dong SL |
| Ref : Pestic Biochem Physiol , 123 :93 , 2015 |
| Abstract : He_2015_Pestic.Biochem.Physiol_123_93 |
| ESTHER : He_2015_Pestic.Biochem.Physiol_123_93 |
| PubMedSearch : He_2015_Pestic.Biochem.Physiol_123_93 |
| PubMedID: 26267057 |
| Gene_locus related to this paper: spoex-g1c2i6 |
| Title : Geranyl acetate esterase controls and regulates the level of geraniol in lemongrass (Cymbopogon flexuosus Nees ex Steud.) mutant cv. GRL-1 leaves - Ganjewala_2009_Z.Naturforsch.C_64_251 |
| Author(s) : Ganjewala D , Luthra R |
| Ref : Z Naturforsch C , 64 :251 , 2009 |
| Abstract : Ganjewala_2009_Z.Naturforsch.C_64_251 |
| ESTHER : Ganjewala_2009_Z.Naturforsch.C_64_251 |
| PubMedSearch : Ganjewala_2009_Z.Naturforsch.C_64_251 |
| PubMedID: 19526721 |
| Title : An integrated process: ester synthesis in an enzymatic membrane reactor and water sorption - Trusek-Holownia_2007_J.Biotechnol_130_47 |
| Author(s) : Trusek-Holownia A , Noworyta A |
| Ref : J Biotechnol , 130 :47 , 2007 |
| Abstract : Trusek-Holownia_2007_J.Biotechnol_130_47 |
| ESTHER : Trusek-Holownia_2007_J.Biotechnol_130_47 |
| PubMedSearch : Trusek-Holownia_2007_J.Biotechnol_130_47 |
| PubMedID: 17434222 |
| Title : Water activity effects on geranyl acetate synthesis catalyzed by novozym in supercritical ethane and in supercritical carbon dioxide - Peres_2003_J.Agric.Food.Chem_51_1884 |
| Author(s) : Peres C , Gomes da Silva MD , Barreiros S |
| Ref : Journal of Agricultural and Food Chemistry , 51 :1884 , 2003 |
| Abstract : Peres_2003_J.Agric.Food.Chem_51_1884 |
| ESTHER : Peres_2003_J.Agric.Food.Chem_51_1884 |
| PubMedSearch : Peres_2003_J.Agric.Food.Chem_51_1884 |
| PubMedID: 12643646 |
| Gene_locus related to this paper: canar-LipB |
| Title : An esterase is involved in geraniol production during palmarosa inflorescence development - Dubey_2003_Phytochemistry_63_257 |
| Author(s) : Dubey VS , Bhalla R , Luthra R |
| Ref : Phytochemistry , 63 :257 , 2003 |
| Abstract : Dubey_2003_Phytochemistry_63_257 |
| ESTHER : Dubey_2003_Phytochemistry_63_257 |
| PubMedSearch : Dubey_2003_Phytochemistry_63_257 |
| PubMedID: 12737976 |
| Title : Lipase-catalyzed synthesis of geranyl acetate in n-hexane with membrane-mediated water removal - Bartling_2001_Biotechnol.Bioeng_75_676 |
| Author(s) : Bartling K , Thompson JU , Pfromm PH , Czermak P , Rezac ME |
| Ref : Biotechnol Bioeng , 75 :676 , 2001 |
| Abstract : Bartling_2001_Biotechnol.Bioeng_75_676 |
| ESTHER : Bartling_2001_Biotechnol.Bioeng_75_676 |
| PubMedSearch : Bartling_2001_Biotechnol.Bioeng_75_676 |
| PubMedID: 11745145 |
| Title : Biotransformation of geranyl acetate to geraniol during palmarosa (Cymbopogon martinii, Roxb. wats. var. motia) inflorescence development - Dubey_2001_Phytochemistry_57_675 |
| Author(s) : Dubey VS , Luthra R |
| Ref : Phytochemistry , 57 :675 , 2001 |
| Abstract : Dubey_2001_Phytochemistry_57_675 |
| ESTHER : Dubey_2001_Phytochemistry_57_675 |
| PubMedSearch : Dubey_2001_Phytochemistry_57_675 |
| PubMedID: 11397433 |
| Title : Bio-degradation of acetates of geraniol, nerol & citronellol by P. incognita: isolation & identification of metabolites - |
| Author(s) : Madyastha KM , Renganathan V |
| Ref : Indian J Biochem Biophys , 20 :136 , 1983 |
| PubMedID: 6671672 |