GYO
General
Type : Analogue of substrate || Lactone || Propionate
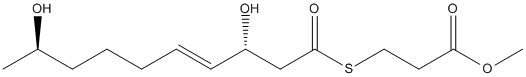
Chemical_Nomenclature : methyl 3-[(E,3R,9R)-3,9-bis(oxidanyl)dec-4-enoyl]sulfanylpropanoate
Canonical SMILES : COC(=O)CCSC(=O)C[C@@H](O)C=CCCC[C@@H](C)O
InChI : InChI=1S\/C14H24O5S\/c1-11(15)6-4-3-5-7-12(16)10-14(18)20-9-8-13(17)19-2\/h5,7,11-12,15-16H,3-4,6,8-10H2,1-2H3\/b7-5+\/t11-,12+\/m1\/s1
InChIKey : ALEAPVQZLMYNNX-BAEOLTKYSA-N
Other name(s) : methyl 3-[(~{E},3~{R},9~{R})-3,9-bis(oxidanyl)dec-4-enoyl]sulfanylpropanoate
MW : 304.40
Formula : C14H24O5S
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Thiohydrolase
References (1)
| Title : A Polyketide Cyclase That Forms Medium-Ring Lactones - Gao_2021_J.Am.Chem.Soc_143_80 |
| Author(s) : Gao DW , Jamieson CS , Wang G , Yan Y , Zhou J , Houk KN , Tang Y |
| Ref : Journal of the American Chemical Society , 143 :80 , 2021 |
| Abstract : Gao_2021_J.Am.Chem.Soc_143_80 |
| ESTHER : Gao_2021_J.Am.Chem.Soc_143_80 |
| PubMedSearch : Gao_2021_J.Am.Chem.Soc_143_80 |
| PubMedID: 33351624 |
| Gene_locus related to this paper: beab2-j4wat9 |