GEUX3
General
Type : Glucuronoyl || Carbohydrate || Glycoside || pNP || Chromogen || Acetate
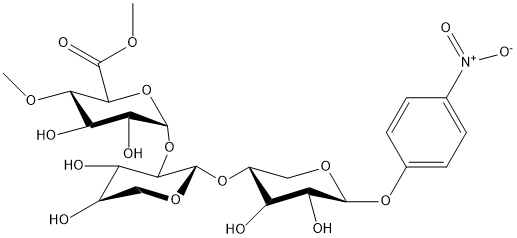
Chemical_Nomenclature : p-nitrophenyl-22-(methyl 4-O-methyl-alpha-D-glucopyranosyluronate)-beta-D-xylobioside
Canonical SMILES : C1(C(COC(C1OC2OC(C(C(C2O)O)OC)C(OC)=O)OC3COC(C(C3O)O)OC4=CC=C(C=C4)[N+](=O)[O-])O)O
InChI : InChI=1S\/C24H33NO17\/c1-35-18-15(29)17(31)23(42-20(18)21(32)36-2)41-19-13(27)11(26)7-37-24(19)40-12-8-38-22(16(30)14(12)28)39-10-5-3-9(4-6-10)25(33)34\/h3-6,11-20,22-24,26-31H,7-8H2,1-2H3
InChIKey : JWOSRBIVNIOCRY-UHFFFAOYSA-N
Other name(s) :
MW : 607.52
Formula : C24H33NO17
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Glucuronoyl_esterase
References (3)
| Title : Efficient activity screening of new glucuronoyl esterases using a pNP-based assay - Madsen_2024_Enzyme.Microb.Technol_178_110444 |
| Author(s) : Madsen MS , Martins PA , Agger JW |
| Ref : Enzyme Microb Technol , 178 :110444 , 2024 |
| Abstract : Madsen_2024_Enzyme.Microb.Technol_178_110444 |
| ESTHER : Madsen_2024_Enzyme.Microb.Technol_178_110444 |
| PubMedSearch : Madsen_2024_Enzyme.Microb.Technol_178_110444 |
| PubMedID: 38581869 |
| Gene_locus related to this paper: 9aphy-a0a2g8sv16 , 9aphy-a0a2g8sk56 , 9fung-ORY47566 , 9fung-KAG4101299 , 9fung-KAG4087973 , aurst-EJD51299 , aurst-EJD54138 , aurst-EJD54133 , cerui-gce |
| Title : Structural and functional investigation of a fungal member of carbohydrate esterase family 15 with potential specificity for rare xylans - Mazurkewich_2023_Acta.Crystallogr.D.Struct.Biol__ |
| Author(s) : Mazurkewich S , Scholzen KC , Brusch RH , Poulsen JCN , Theibich Y , Huttner S , Olsson L , Larsbrink J , Lo Leggio L |
| Ref : Acta Crystallographica D Struct Biol , : , 2023 |
| Abstract : Mazurkewich_2023_Acta.Crystallogr.D.Struct.Biol__ |
| ESTHER : Mazurkewich_2023_Acta.Crystallogr.D.Struct.Biol__ |
| PubMedSearch : Mazurkewich_2023_Acta.Crystallogr.D.Struct.Biol__ |
| PubMedID: 37227091 |
| Gene_locus related to this paper: 9pleo-LfCE15C |
| Title : beta-Glucuronidase-coupled assays of glucuronoyl esterases - Franova_2016_Anal.Biochem_510_114 |
| Author(s) : Franova L , Puchart V , Biely P |
| Ref : Analytical Biochemistry , 510 :114 , 2016 |
| Abstract : Franova_2016_Anal.Biochem_510_114 |
| ESTHER : Franova_2016_Anal.Biochem_510_114 |
| PubMedSearch : Franova_2016_Anal.Biochem_510_114 |
| PubMedID: 27452816 |