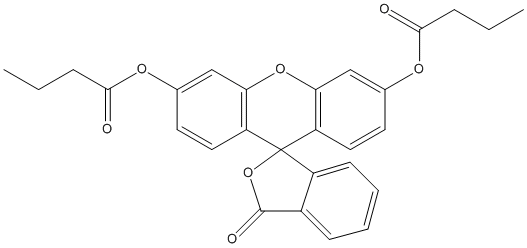
Fluorescein-dibutyrate
General
Type : Fluorescent Probe || Benzofuran || Xanthene
Chemical_Nomenclature : (6'-butanoyloxy-3-oxospiro[2-benzofuran-1,9'-xanthene]-3'-yl) butanoate
Canonical SMILES : CCCC(=O)OC1=CC2=C(C=C1)C3(C4=C(O2)C=C(C=C4)OC(=O)CCC)C5=CC=CC=C5C(=O)O3
InChI : InChI=1S\/C28H24O7\/c1-3-7-25(29)32-17-11-13-21-23(15-17)34-24-16-18(33-26(30)8-4-2)12-14-22(24)28(21)20-10-6-5-9-19(20)27(31)35-28\/h5-6,9-16H,3-4,7-8H2,1-2H3
InChIKey : CGMHIZMGYHYHML-UHFFFAOYSA-N
Other name(s) : Fluorescein, dibutyrate, UNII-ONT4U528WD, FDBu, Fluorescein dibutyrate, dibutyryl fluorescine
Target
Families : Hormone-sensitive_lipase_like, Lipase_3, Carb_B_Chordata
References (7)
| Title : Development and validation of an improved diced electrophoresis gel assay cutter-plate system for enzymomics studies - Komatsu_2019_Biochim.Biophys.Acta.Proteins.Proteom_1867_82 |
| Author(s) : Komatsu T , Shimoda M , Kawamura Y , Urano Y , Nagano T |
| Ref : Biochimica & Biophysica Acta Proteins Proteom , 1867 :82 , 2019 |
| Abstract : Komatsu_2019_Biochim.Biophys.Acta.Proteins.Proteom_1867_82 |
| ESTHER : Komatsu_2019_Biochim.Biophys.Acta.Proteins.Proteom_1867_82 |
| PubMedSearch : Komatsu_2019_Biochim.Biophys.Acta.Proteins.Proteom_1867_82 |
| PubMedID: 29928991 |
| Title : Identification of Tissue-Restricted Bioreaction Suitable for in Vivo Targeting by Fluorescent Substrate Library-Based Enzyme Discovery - Yoshioka_2015_J.Am.Chem.Soc_137_12187 |
| Author(s) : Yoshioka K , Komatsu T , Nakada A , Onagi J , Kuriki Y , Kawaguchi M , Terai T , Ueno T , Hanaoka K , Nagano T , Urano Y |
| Ref : Journal of the American Chemical Society , 137 :12187 , 2015 |
| Abstract : Yoshioka_2015_J.Am.Chem.Soc_137_12187 |
| ESTHER : Yoshioka_2015_J.Am.Chem.Soc_137_12187 |
| PubMedSearch : Yoshioka_2015_J.Am.Chem.Soc_137_12187 |
| PubMedID: 26360463 |
| Gene_locus related to this paper: human-CES2 |
| Title : Creation of Rhizopus oryzae lipase having a unique oxyanion hole by combinatorial mutagenesis in the lid domain - Shiraga_2005_Appl.Microbiol.Biotechnol_68_779 |
| Author(s) : Shiraga S , Ishiguro M , Fukami H , Nakao M , Ueda M |
| Ref : Applied Microbiology & Biotechnology , 68 :779 , 2005 |
| Abstract : Shiraga_2005_Appl.Microbiol.Biotechnol_68_779 |
| ESTHER : Shiraga_2005_Appl.Microbiol.Biotechnol_68_779 |
| PubMedSearch : Shiraga_2005_Appl.Microbiol.Biotechnol_68_779 |
| PubMedID: 15729555 |
| Gene_locus related to this paper: rhidl-lipas |
| Title : Lipid content and lipoprotein lipase activity in skeletal muscle of lactating and weaned rats - Del Prado_1993_J.Lipid.Res_34_1115 |
| Author(s) : Del Prado M , Ramos R , Hernandez-Montes H , Villalpando S |
| Ref : J Lipid Res , 34 :1115 , 1993 |
| Abstract : Del Prado_1993_J.Lipid.Res_34_1115 |
| ESTHER : Del Prado_1993_J.Lipid.Res_34_1115 |
| PubMedSearch : Del Prado_1993_J.Lipid.Res_34_1115 |
| PubMedID: 8371059 |
| Title : An improved method for the determination of pregerminated grains in barley - Jensen_1983_Carlsberg Res Commun_48_1 |
| Author(s) : Jensen SA , Heltved F |
| Ref : Carlsberg Research Communications , 48 :1 , 1983 |
| Abstract : Jensen_1983_Carlsberg Res Commun_48_1 |
| ESTHER : Jensen_1983_Carlsberg Res Commun_48_1 |
| PubMedSearch : Jensen_1983_Carlsberg Res Commun_48_1 |
| PubMedID: |
| Title : Visualization of enzyme activity in germinating cereal seeds using a lipase sensitive fluorochrome - Jensen_1982_Carlsberg Res Commun_47_297 |
| Author(s) : Jensen SA , Heltved F |
| Ref : Carlsberg Research Communications , 47 :297 , 1982 |
| Abstract : Jensen_1982_Carlsberg Res Commun_47_297 |
| ESTHER : Jensen_1982_Carlsberg Res Commun_47_297 |
| PubMedSearch : Jensen_1982_Carlsberg Res Commun_47_297 |
| PubMedID: |
| Title : The fluorimetric assay of a rat adipose tisssue lipase (fluorescein dibutyrate esterase) - |
| Author(s) : Sapira JD , Tyrrell EM , Klaniecki T , Shapiro AP |
| Ref : Enzymologia , 30 :97 , 1966 |
| PubMedID: 6005318 |