Fluazifop-butyl
General
Type : Herbicide || Trifluoro || Pyridine
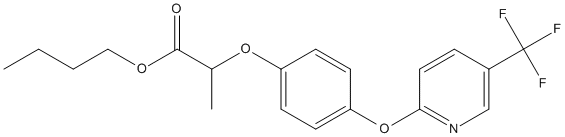
Chemical_Nomenclature : butyl 2-[4-[5-(trifluoromethyl)pyridin-2-yl]oxyphenoxy]propanoate
Canonical SMILES : CCCCOC(=O)C(C)OC1=CC=C(C=C1)OC2=NC=C(C=C2)C(F)(F)F
InChI : InChI=1S\/C19H20F3NO4\/c1-3-4-11-25-18(24)13(2)26-15-6-8-16(9-7-15)27-17-10-5-14(12-23-17)19(20,21)22\/h5-10,12-13H,3-4,11H2,1-2H3
InChIKey : VAIZTNZGPYBOGF-UHFFFAOYSA-N
Other name(s) : Fluazifop butyl, Fusilade, Halokon, Onecide, SCHEMBL54222, CHEBI:5097, CHEMBL449397, CHEBI:132963
Target
Families : Carb_B_Chordata
References (3)
| Title : Human xenobiotic metabolizing esterases in liver and blood - McCracken_1993_Biochem.Pharmacol_46_1125 |
| Author(s) : McCracken NW , Blain PG , Williams FM |
| Ref : Biochemical Pharmacology , 46 :1125 , 1993 |
| Abstract : McCracken_1993_Biochem.Pharmacol_46_1125 |
| ESTHER : McCracken_1993_Biochem.Pharmacol_46_1125 |
| PubMedSearch : McCracken_1993_Biochem.Pharmacol_46_1125 |
| PubMedID: 8216361 |
| Title : Peripheral esterases in the rat: effects of classical inducers - McCracken_1993_Chem.Biol.Interact_87_183 |
| Author(s) : McCracken NW , Blain PG , Williams FM |
| Ref : Chemico-Biological Interactions , 87 :183 , 1993 |
| Abstract : McCracken_1993_Chem.Biol.Interact_87_183 |
| ESTHER : McCracken_1993_Chem.Biol.Interact_87_183 |
| PubMedSearch : McCracken_1993_Chem.Biol.Interact_87_183 |
| PubMedID: 8343974 |
| Title : Esterase activity in rat hepatocytes - Williams_1991_Biochem.Pharmacol_41_527 |
| Author(s) : Williams FM , Mutch E , Blain PG |
| Ref : Biochemical Pharmacology , 41 :527 , 1991 |
| Abstract : Williams_1991_Biochem.Pharmacol_41_527 |
| ESTHER : Williams_1991_Biochem.Pharmacol_41_527 |
| PubMedSearch : Williams_1991_Biochem.Pharmacol_41_527 |
| PubMedID: 1997002 |