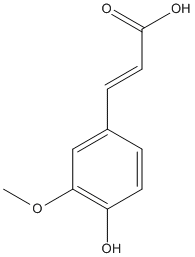
Ferulic-acid
General
Type : Acrylate || Feruloyl || Hydroxycinnamic-ester
Chemical_Nomenclature : (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid
Canonical SMILES : COC1=C(C=CC(=C1)C=CC(=O)O)O
InChI : InChI=1S\/C10H10O4\/c1-14-9-6-7(2-4-8(9)11)3-5-10(12)13\/h2-6,11H,1H3,(H,12,13)\/b5-3+
InChIKey : KSEBMYQBYZTDHS-HWKANZROSA-N
Other name(s) : Ferulic acid, Trans-Ferulic Acid, 4-Hydroxy-3-methoxycinnamic acid, Trans-4-Hydroxy-3-methoxycinnamic acid, 3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid, Methyl 4-hydroxy-3-methoxycinnamate, FER
Target
Families : A85-Feruloyl-Esterase, FAE-Bacterial-promiscuous, Lipase_3, Tannase
References (11)
| Title : Crystal structure of the feruloyl esterase from Lentilactobacillus buchneri reveals a novel homodimeric state - Kasmaei_2022_Front.Microbiol_13_1050160 |
| Author(s) : Kasmaei KM , Kalyani DC , Reichenbach T , Jimenez-Quero A , Vilaplana F , Divne C |
| Ref : Front Microbiol , 13 :1050160 , 2022 |
| Abstract : Kasmaei_2022_Front.Microbiol_13_1050160 |
| ESTHER : Kasmaei_2022_Front.Microbiol_13_1050160 |
| PubMedSearch : Kasmaei_2022_Front.Microbiol_13_1050160 |
| PubMedID: 36569051 |
| Gene_locus related to this paper: lenbu-d7ru28 |
| Title : Therapeutic Potential of Ferulic Acid in Alzheimer's Disease - Turkez_2021_Curr.Drug.Deliv__ |
| Author(s) : Turkez H , Arslan ME , Barboza JN , Kahraman CY , de Sousa DP , Mardinoglu A |
| Ref : Curr Drug Deliv , : , 2021 |
| Abstract : Turkez_2021_Curr.Drug.Deliv__ |
| ESTHER : Turkez_2021_Curr.Drug.Deliv__ |
| PubMedSearch : Turkez_2021_Curr.Drug.Deliv__ |
| PubMedID: 34963433 |
| Title : Lipase-catalyzed esterification of ferulic acid with lauryl alcohol in ionic liquids and antibacterial properties in vitro against three food-related bacteria - Shi_2017_Food.Chem_220_249 |
| Author(s) : Shi YG , Wu Y , Lu XY , Ren YP , Wang Q , Zhu CM , Yu D , Wang H |
| Ref : Food Chem , 220 :249 , 2017 |
| Abstract : Shi_2017_Food.Chem_220_249 |
| ESTHER : Shi_2017_Food.Chem_220_249 |
| PubMedSearch : Shi_2017_Food.Chem_220_249 |
| PubMedID: 27855896 |
| Title : Contributions of a unique beta-clamp to substrate recognition illuminates the molecular basis of exolysis in ferulic acid esterases - Gruninger_2016_Biochem.J_473_839 |
| Author(s) : Gruninger RJ , Cote C , McAllister TA , Abbott DW |
| Ref : Biochemical Journal , 473 :839 , 2016 |
| Abstract : Gruninger_2016_Biochem.J_473_839 |
| ESTHER : Gruninger_2016_Biochem.J_473_839 |
| PubMedSearch : Gruninger_2016_Biochem.J_473_839 |
| PubMedID: 27026397 |
| Gene_locus related to this paper: 9fung-f2ycb6 |
| Title : Heterologous production of a feruloyl esterase from Pleurotus sapidus synthesizing feruloyl-saccharide esters - Kelle_2016_Biotechnol.Appl.Biochem_63_852 |
| Author(s) : Kelle S , Nieter A , Krings U , Zelena K , Linke D , Berger RG |
| Ref : Biotechnol Appl Biochem , 63 :852 , 2016 |
| Abstract : Kelle_2016_Biotechnol.Appl.Biochem_63_852 |
| ESTHER : Kelle_2016_Biotechnol.Appl.Biochem_63_852 |
| PubMedSearch : Kelle_2016_Biotechnol.Appl.Biochem_63_852 |
| PubMedID: 26272349 |
| Gene_locus related to this paper: 9agar-d5grp3 |
| Title : An inserted alpha\/beta subdomain shapes the catalytic pocket of Lactobacillus johnsonii cinnamoyl esterase - Lai_2011_PLoS.One_6_e23269 |
| Author(s) : Lai KK , Stogios PJ , Vu C , Xu X , Cui H , Molloy S , Savchenko A , Yakunin A , Gonzalez CF |
| Ref : PLoS ONE , 6 :e23269 , 2011 |
| Abstract : Lai_2011_PLoS.One_6_e23269 |
| ESTHER : Lai_2011_PLoS.One_6_e23269 |
| PubMedSearch : Lai_2011_PLoS.One_6_e23269 |
| PubMedID: 21876742 |
| Gene_locus related to this paper: lacjo-q74hk5 |
| Title : Probing the determinants of substrate specificity of a feruloyl esterase, AnFaeA, from Aspergillus niger - Faulds_2005_Febs.J_272_4362 |
| Author(s) : Faulds CB , Molina R , Gonzalez R , Husband F , Juge N , Sanz-Aparicio J , Hermoso JA |
| Ref : Febs J , 272 :4362 , 2005 |
| Abstract : Faulds_2005_Febs.J_272_4362 |
| ESTHER : Faulds_2005_Febs.J_272_4362 |
| PubMedSearch : Faulds_2005_Febs.J_272_4362 |
| PubMedID: 16128806 |
| Gene_locus related to this paper: aspni-FAEA |
| Title : Structure of a feruloyl esterase from Aspergillus niger - McAuley_2004_Acta.Crystallogr.D.Biol.Crystallogr_60_878 |
| Author(s) : McAuley KE , Svendsen A , Patkar SA , Wilson KS |
| Ref : Acta Crystallographica D Biol Crystallogr , 60 :878 , 2004 |
| Abstract : McAuley_2004_Acta.Crystallogr.D.Biol.Crystallogr_60_878 |
| ESTHER : McAuley_2004_Acta.Crystallogr.D.Biol.Crystallogr_60_878 |
| PubMedSearch : McAuley_2004_Acta.Crystallogr.D.Biol.Crystallogr_60_878 |
| PubMedID: 15103133 |
| Gene_locus related to this paper: aspni-FAEA |
| Title : The Structure of the Feruloyl Esterase Module of Xylanase 10B from Clostridium thermocellum Provides Insights into Substrate Recognition - Prates_2001_Structure_9_1183 |
| Author(s) : Prates JA , Tarbouriech N , Charnock SJ , Fontes CM , Ferreira LM , Davies GJ |
| Ref : Structure , 9 :1183 , 2001 |
| Abstract : Prates_2001_Structure_9_1183 |
| ESTHER : Prates_2001_Structure_9_1183 |
| PubMedSearch : Prates_2001_Structure_9_1183 |
| PubMedID: 11738044 |
| Gene_locus related to this paper: clotm-xyny |
| Title : Structural Basis for the Substrate Specificity of the Feruloyl Esterase Domain of the Cellulosomal Xylanase Z from Clostridium Thermocellum - Schubot_2001_Biochemistry_40_12524 |
| Author(s) : Schubot FD , Kataeva IA , Blum DL , Shah AK , Ljungdahl LG , Rose JP , Wang BC |
| Ref : Biochemistry , 40 :12524 , 2001 |
| Abstract : Schubot_2001_Biochemistry_40_12524 |
| ESTHER : Schubot_2001_Biochemistry_40_12524 |
| PubMedSearch : Schubot_2001_Biochemistry_40_12524 |
| PubMedID: 11601976 |
| Gene_locus related to this paper: clotm-xynz |
| Title : Methyl phenylalkanoates as substrates to probe the active sites of esterases - Kroon_1997_Eur.J.Biochem_248_245 |
| Author(s) : Kroon PA , Faulds CB , Brezillon C , Williamson G |
| Ref : European Journal of Biochemistry , 248 :245 , 1997 |
| Abstract : Kroon_1997_Eur.J.Biochem_248_245 |
| ESTHER : Kroon_1997_Eur.J.Biochem_248_245 |
| PubMedSearch : Kroon_1997_Eur.J.Biochem_248_245 |
| PubMedID: 9310385 |
| Gene_locus related to this paper: aspng-faeb , aspni-FAEA |