Ethyl-oleate
General
Type : Oleate || Alkyl ester
Chemical_Nomenclature : ethyl (Z)-octadec-9-enoate
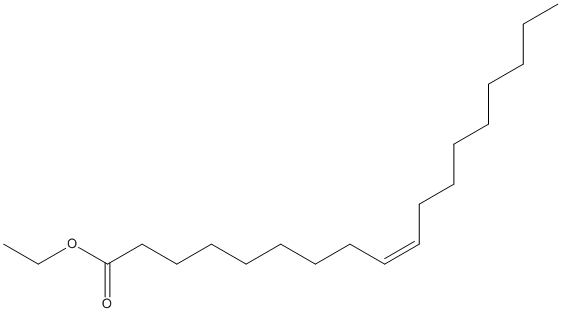
Canonical SMILES : CCCCCCCCC=CCCCCCCCC(=O)OCC
InChI : InChI=1S\/C20H38O2\/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22-4-2\/h11-12H,3-10,13-19H2,1-2H3\/b12-11-
InChIKey : LVGKNOAMLMIIKO-QXMHVHEDSA-N
Other name(s) : Ethyl oleate, Oleic acid ethyl ester, Ethyl cis-9-octadecenoate, Oleic acid, ethyl ester, CHEBI:84940
Target
Families : Fungal-Bact_LIP
References (3)
| Title : Inhibition of CpLIP2 Lipase Hydrolytic Activity by Four Flavonols (Galangin, Kaempferol, Quercetin, Myricetin) Compared to Orlistat and Their Binding Mechanisms Studied by Quenching of Fluorescence - Nasri_2019_Molecules_24_ |
| Author(s) : Nasri R , Bidel LPR , Rugani N , Perrier V , Carriere F , Dubreucq E , Jay-Allemand C |
| Ref : Molecules , 24 : , 2019 |
| Abstract : Nasri_2019_Molecules_24_ |
| ESTHER : Nasri_2019_Molecules_24_ |
| PubMedSearch : Nasri_2019_Molecules_24_ |
| PubMedID: 31398944 |
| Gene_locus related to this paper: canpa-LIP2 |
| Title : Continuous ethyl oleate synthesis by lipases produced by solid-state fermentation by Rhizopus microsporus - Martinez-Ruiz_2018_Bioresour.Technol_265_52 |
| Author(s) : Martinez-Ruiz A , Tovar-Castro L , Garcia HS , Saucedo-Castaneda G , Favela-Torres E |
| Ref : Bioresour Technol , 265 :52 , 2018 |
| Abstract : Martinez-Ruiz_2018_Bioresour.Technol_265_52 |
| ESTHER : Martinez-Ruiz_2018_Bioresour.Technol_265_52 |
| PubMedSearch : Martinez-Ruiz_2018_Bioresour.Technol_265_52 |
| PubMedID: 29879651 |
| Title : Peculiar features of four enzymes of the CaLA superfamily in aqueous media: Differences in substrate specificities and abilities to catalyze alcoholysis - Neang_2013_J.Mol.Catal.B.Enzym_94_36 |
| Author(s) : Neang PM , Subileau M , Perrier V , Dubreucq E |
| Ref : J Mol Catal B Enzym , 94 :36 , 2013 |
| Abstract : Neang_2013_J.Mol.Catal.B.Enzym_94_36 |
| ESTHER : Neang_2013_J.Mol.Catal.B.Enzym_94_36 |
| PubMedSearch : Neang_2013_J.Mol.Catal.B.Enzym_94_36 |
| PubMedID: |
| Gene_locus related to this paper: aspor-q2uix9 , canan-lipasA , canpa-LIP2 , cantt-c5mag0 |