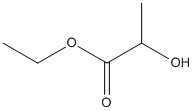
Ethyl-lactate
General
Type : Alkyl ester || Propionate
Chemical_Nomenclature : ethyl 2-hydroxypropanoate
Canonical SMILES : CCOC(=O)C(C)O
InChI : InChI=1S\/C5H10O3\/c1-3-8-5(7)4(2)6\/h4,6H,3H2,1-2H3
InChIKey : LZCLXQDLBQLTDK-UHFFFAOYSA-N
Other name(s) : Ethyl 2-hydroxypropanoate, Actylol, Acytol, Solactol
Target
Families : Polyesterase-lipase-cutinase
References (4)
| Title : Efficient synthesis of enantiomeric ethyl lactate by Candida antarctica lipase B (CALB)-displaying yeasts - Inaba_2009_Appl.Microbiol.Biotechnol_83_859 |
| Author(s) : Inaba C , Maekawa K , Morisaka H , Kuroda K , Ueda M |
| Ref : Applied Microbiology & Biotechnology , 83 :859 , 2009 |
| Abstract : Inaba_2009_Appl.Microbiol.Biotechnol_83_859 |
| ESTHER : Inaba_2009_Appl.Microbiol.Biotechnol_83_859 |
| PubMedSearch : Inaba_2009_Appl.Microbiol.Biotechnol_83_859 |
| PubMedID: 19288094 |
| Gene_locus related to this paper: canar-LipB |
| Title : Enzymatic synthesis of galactosyl lactic ethyl ester and its polymer for use as biomaterials - Jia_2007_J.Biotechnol_132_314 |
| Author(s) : Jia H , Wang P |
| Ref : J Biotechnol , 132 :314 , 2007 |
| Abstract : Jia_2007_J.Biotechnol_132_314 |
| ESTHER : Jia_2007_J.Biotechnol_132_314 |
| PubMedSearch : Jia_2007_J.Biotechnol_132_314 |
| PubMedID: 17689799 |
| Title : Inhibition of pig liver esterase by trifluoromethyl ketones: modulators of the catalytic reaction alter inhibition kinetics - Allen_1989_Biochemistry_28_135 |
| Author(s) : Allen KN , Abeles RH |
| Ref : Biochemistry , 28 :135 , 1989 |
| Abstract : Allen_1989_Biochemistry_28_135 |
| ESTHER : Allen_1989_Biochemistry_28_135 |
| PubMedSearch : Allen_1989_Biochemistry_28_135 |
| PubMedID: 2706239 |