E-2-hexenyl-acetate
General
Type : Acetate || Plant volatile || Natural
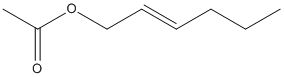
Chemical_Nomenclature : [(E)-hex-2-enyl] acetate
Canonical SMILES : CCCC=CCOC(=O)C
InChI : InChI=1S\/C8H14O2\/c1-3-4-5-6-7-10-8(2)9\/h5-6H,3-4,7H2,1-2H3\/b6-5+
InChIKey : HRHOWZHRCRZVCU-AATRIKPKSA-N
Other name(s) : trans-2-Hexenyl acetate, 2-Hexenyl acetate, trans-Hex-2-enyl acetate, (E)-2-hexenyl acetate, XDV436N45E, CHEMBL2251453
Target
Families : Plant_carboxylesterase, Carb_B_Arthropoda
References (3)
| Title : The Functional Characterization of Carboxylesterases Involved in the Degradation of Volatile Esters Produced in Strawberry Fruits - Zhang_2022_Int.J.Mol.Sci_24_383 |
| Author(s) : Zhang L , Zhou K , Wang M , Li R , Dai X , Liu Y , Jiang X , Xia T , Gao L |
| Ref : Int J Mol Sci , 24 :383 , 2022 |
| Abstract : Zhang_2022_Int.J.Mol.Sci_24_383 |
| ESTHER : Zhang_2022_Int.J.Mol.Sci_24_383 |
| PubMedSearch : Zhang_2022_Int.J.Mol.Sci_24_383 |
| PubMedID: 36613824 |
| Gene_locus related to this paper: frave-FanCXE17 , frave-FanCXE19 , frave-FanCXE3 , frave-FanCXE2 , fraan-FaTA |
| Title : Peach Carboxylesterase PpCXE1 Is Associated with Catabolism of Volatile Esters - Cao_2019_J.Agric.Food.Chem_67_5189 |
| Author(s) : Cao X , Xie K , Duan W , Zhu Y , Liu M , Chen K , Klee H , Zhang B |
| Ref : Journal of Agricultural and Food Chemistry , 67 :5189 , 2019 |
| Abstract : Cao_2019_J.Agric.Food.Chem_67_5189 |
| ESTHER : Cao_2019_J.Agric.Food.Chem_67_5189 |
| PubMedSearch : Cao_2019_J.Agric.Food.Chem_67_5189 |
| PubMedID: 30997798 |
| Gene_locus related to this paper: prupe-m5w2v0 , prupe-m5vkk2 , prupe-m5vq13 |
| Title : An antenna-biased carboxylesterase is specifically active to plant volatiles in Spodoptera exigua - He_2015_Pestic.Biochem.Physiol_123_93 |
| Author(s) : He P , Zhang YN , Yang K , Li ZQ , Dong SL |
| Ref : Pestic Biochem Physiol , 123 :93 , 2015 |
| Abstract : He_2015_Pestic.Biochem.Physiol_123_93 |
| ESTHER : He_2015_Pestic.Biochem.Physiol_123_93 |
| PubMedSearch : He_2015_Pestic.Biochem.Physiol_123_93 |
| PubMedID: 26267057 |
| Gene_locus related to this paper: spoex-g1c2i6 |