Dipalmitoylphosphatidylcholine
General
Type : Phospholipid || Phosphatidylcholine || Diacylglycerol || Trimethylammonium || Choline ester || Hexadecanoate || Palmitate || Surfactant
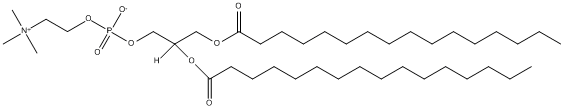
Chemical_Nomenclature : 2,3-di(hexadecanoyloxy)propyl 2-(trimethylazaniumyl)ethyl phosphate
Canonical SMILES : CCCCCCCCCCCCCCCC(=O)OCC(COP(=O)([O-])OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC
InChI : InChI=1S\/C40H80NO8P\/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-39(42)46-36-38(37-48-50(44,45)47-35-34-41(3,4)5)49-40(43)33-31-29-27-25-23-21-19-17-15-13-11-9-7-2\/h38H,6-37H2,1-5H3
InChIKey : KILNVBDSWZSGLL-UHFFFAOYSA-N
Other name(s) : Dipalmitoyl phosphatidylcholine, Dipalmitoyl phosphatidylcholine[PC], DPPC, Colfosceril palmitate, DPPC (phosphatide), Coatsome MC 6060, 1,2-Dipalmitoyllecithin, 1,2-Dipalmitoyl-L-lecithin, 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine, 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine, DiPalmitoylphosphatidylcholine
MW : 734.03
Formula : C40H80NO8P
CAS_number : 2644-64-6 || 2797-68-4 || 63-89-8
PubChem :
UniChem :
Iuphar :
Target
Families : PlaB, PC-sterol_acyltransferase
References (2)
| Title : Phospholipase PlaB of Legionella pneumophila represents a novel lipase family: protein residues essential for lipolytic activity, substrate specificity, and hemolysis - Bender_2009_J.Biol.Chem_284_27185 |
| Author(s) : Bender J , Rydzewski K , Broich M , Schunder E , Heuner K , Flieger A |
| Ref : Journal of Biological Chemistry , 284 :27185 , 2009 |
| Abstract : Bender_2009_J.Biol.Chem_284_27185 |
| ESTHER : Bender_2009_J.Biol.Chem_284_27185 |
| PubMedSearch : Bender_2009_J.Biol.Chem_284_27185 |
| PubMedID: 19640837 |
| Gene_locus related to this paper: legpn-i7i328 , legsp-a3fmk8 |
| Title : The stability of liposomes in vitro to pH, bile salts and pancreatic lipase - Rowland_1980_Biochim.Biophys.Acta_620_400 |
| Author(s) : Rowland RN , Woodley JF |
| Ref : Biochimica & Biophysica Acta , 620 :400 , 1980 |
| Abstract : Rowland_1980_Biochim.Biophys.Acta_620_400 |
| ESTHER : Rowland_1980_Biochim.Biophys.Acta_620_400 |
| PubMedSearch : Rowland_1980_Biochim.Biophys.Acta_620_400 |
| PubMedID: 7016185 |