Dimethyl-fumarate
General
Type : Drug || Pro-Drug || Not A\/B H target
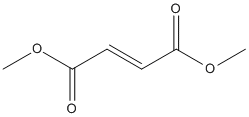
Chemical_Nomenclature : dimethyl (E)-but-2-enedioate
Canonical SMILES : COC(=O)C=CC(=O)OC
InChI : InChI=1S\/C6H8O4\/c1-9-5(7)3-4-6(8)10-2\/h3-4H,1-2H3\/b4-3+
InChIKey : LDCRTTXIJACKKU-ONEGZZNKSA-N
Other name(s) : Dimethyl fumarate, Tecfidera, Dimethylfumarate, Methyl fumarate, SCHEMBL41835, SCHEMBL41836, CHEMBL2107333, ZINC3843378, DB08908
Target
Families : Carb_B_Chordata
References (4)
| Title : A multi-target directed ligands strategy for the treatment of Alzheimer's disease: Dimethyl fumarate plus Tranilast modified Dithiocarbate as AChE inhibitor and Nrf2 activator - Guo_2022_Eur.J.Med.Chem_242_114630 |
| Author(s) : Guo J , Cheng M , Liu P , Cao D , Luo J , Wan Y , Fang Y , Jin Y , Xie SS , Liu J |
| Ref : Eur Journal of Medicinal Chemistry , 242 :114630 , 2022 |
| Abstract : Guo_2022_Eur.J.Med.Chem_242_114630 |
| ESTHER : Guo_2022_Eur.J.Med.Chem_242_114630 |
| PubMedSearch : Guo_2022_Eur.J.Med.Chem_242_114630 |
| PubMedID: 35987018 |
| Title : Alcohol inhibits the metabolism of dimethyl fumarate to the active metabolite responsible for decreasing relapse frequency in the treatment of multiple sclerosis - Yang_2022_PLoS.One_17_e0278111 |
| Author(s) : Yang B , Parker RB , Meibohm B , Temrikar ZH , Srivastava A , Laizure SC |
| Ref : PLoS ONE , 17 :e0278111 , 2022 |
| Abstract : Yang_2022_PLoS.One_17_e0278111 |
| ESTHER : Yang_2022_PLoS.One_17_e0278111 |
| PubMedSearch : Yang_2022_PLoS.One_17_e0278111 |
| PubMedID: 36441753 |
| Title : A Sensitization-Free Dimethyl Fumarate Prodrug, Isosorbide Di-(Methyl Fumarate), Provides a Topical Treatment Candidate for Psoriasis - Bojanowski_2021_JID.Innov_1_100040 |
| Author(s) : Bojanowski K , Ibeji CU , Singh P , Swindell WR , Chaudhuri RK |
| Ref : JID Innov , 1 :100040 , 2021 |
| Abstract : Bojanowski_2021_JID.Innov_1_100040 |
| ESTHER : Bojanowski_2021_JID.Innov_1_100040 |
| PubMedSearch : Bojanowski_2021_JID.Innov_1_100040 |
| PubMedID: 34909741 |
| Title : Dimethyl fumarate attenuates 2-VO-induced vascular dementia via activating the Nrf2 signaling pathway in rats - Dhaliwal_2021_Inflammopharmacology__ |
| Author(s) : Dhaliwal N , Dhaliwal J , Singh A , Chopra K |
| Ref : Inflammopharmacology , : , 2021 |
| Abstract : Dhaliwal_2021_Inflammopharmacology__ |
| ESTHER : Dhaliwal_2021_Inflammopharmacology__ |
| PubMedSearch : Dhaliwal_2021_Inflammopharmacology__ |
| PubMedID: 33459879 |