Diethyl-adipate
General
Type : Fatty acid ester
Chemical_Nomenclature :
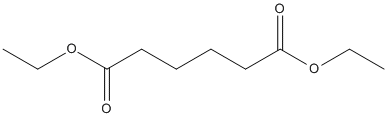
Canonical SMILES :
InChI :
InChIKey :
Other name(s) : Diethyl adipate, Diethyl hexanedioate, Ethyl adipate, Hexanedioic acid, diethyl ester, SCHEMBL50610, CHEBI:34697, ZINC166673
Target
Families : Cutinase, Canar_LipB
References (5)
| Title : Cutinase ACut2 from Blastobotrysraffinosifermentans for the Selective Desymmetrization of the Symmetric Diester Diethyl Adipate to the Monoester Monoethyl Adipate - Rauter_2022_Microorganisms_10_1316 |
| Author(s) : Rauter M , Nietz D , Kunze G |
| Ref : Microorganisms , 10 : , 2022 |
| Abstract : Rauter_2022_Microorganisms_10_1316 |
| ESTHER : Rauter_2022_Microorganisms_10_1316 |
| PubMedSearch : Rauter_2022_Microorganisms_10_1316 |
| PubMedID: 35889035 |
| Gene_locus related to this paper: blaad-a0a060t5x1 , blaad-a0a060t512 , blaad-a0a060t6b2 |
| Title : Effects of Methyl Branching on the Properties and Performance of Furandioate-Adipate Copolyesters of Bio-Based Secondary Diols - Little_2020_ACS.Sustain.Chem.Eng_8_14471 |
| Author(s) : Little A , Pellis A , Comerford JW , Naranjo-Valles E , Hafezi N , Mascal M , Farmer TJ |
| Ref : ACS Sustain Chem Eng , 8 :14471 , 2020 |
| Abstract : Little_2020_ACS.Sustain.Chem.Eng_8_14471 |
| ESTHER : Little_2020_ACS.Sustain.Chem.Eng_8_14471 |
| PubMedSearch : Little_2020_ACS.Sustain.Chem.Eng_8_14471 |
| PubMedID: 33014637 |
| Title : Enzymatic Polycondensation of 1,6-Hexanediol and Diethyl Adipate: A Statistical Approach Predicting the Key-Parameters in Solution and in Bulk - Nasr_2020_Polymers.(Basel)_12_ |
| Author(s) : Nasr K , Meimoun J , Favrelle-Huret A , Winter J , Raquez JM , Zinck P |
| Ref : Polymers (Basel) , 12 : , 2020 |
| Abstract : Nasr_2020_Polymers.(Basel)_12_ |
| ESTHER : Nasr_2020_Polymers.(Basel)_12_ |
| PubMedSearch : Nasr_2020_Polymers.(Basel)_12_ |
| PubMedID: 32847050 |
| Gene_locus related to this paper: canar-LipB |
| Title : Three New Cutinases from the Yeast Arxula adeninivorans That Are Suitable for Biotechnological Applications - Bischoff_2015_Appl.Environ.Microbiol_81_5497 |
| Author(s) : Bischoff F , Litwinska K , Cordes A , Baronian K , Bode R , Schauer F , Kunze G |
| Ref : Applied Environmental Microbiology , 81 :5497 , 2015 |
| Abstract : Bischoff_2015_Appl.Environ.Microbiol_81_5497 |
| ESTHER : Bischoff_2015_Appl.Environ.Microbiol_81_5497 |
| PubMedSearch : Bischoff_2015_Appl.Environ.Microbiol_81_5497 |
| PubMedID: 26048925 |
| Gene_locus related to this paper: blaad-a0a060t5x1 , blaad-a0a060t512 , blaad-a0a060t6b2 |
| Title : Degradation of malathion, in aqueous extracts of asparagus (Asparagus officinalis) - Okamoto_2004_J.Agric.Food.Chem_52_5919 |
| Author(s) : Okamoto Y , Shibamoto T |
| Ref : Journal of Agricultural and Food Chemistry , 52 :5919 , 2004 |
| Abstract : Okamoto_2004_J.Agric.Food.Chem_52_5919 |
| ESTHER : Okamoto_2004_J.Agric.Food.Chem_52_5919 |
| PubMedSearch : Okamoto_2004_J.Agric.Food.Chem_52_5919 |
| PubMedID: 15366843 |