Deltamethrin
General
Type : pyrethroid || Insecticide || Phenoxyphenyl || Cyanide || Cyclopropane
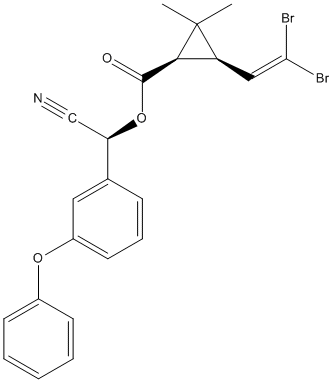
Chemical_Nomenclature : [(S)-cyano-(3-phenoxyphenyl)methyl] (1R,3R)-3-(2,2-dibromoethenyl)-2,2-dimethylcyclopropane-1-carboxylate
Canonical SMILES : CC1(C(C1C(=O)OC(C#N)C2=CC(=CC=C2)OC3=CC=CC=C3)C=C(Br)Br)C
InChI : InChI=1S\/C22H19Br2NO3\/c1-22(2)17(12-19(23)24)20(22)21(26)28-18(13-25)14-7-6-10-16(11-14)27-15-8-4-3-5-9-15\/h3-12,17-18,20H,1-2H3\/t17-,18+,20-\/m0\/s1
InChIKey : OWZREIFADZCYQD-NSHGMRRFSA-N
Other name(s) : Decamethrin, Butox, Decis, K-Othrine, K-Othrin
MW : 505.19
Formula : C22H19Br2NO3
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
References (13)
| Title : Enhancement degradation efficiency of pyrethroid-degrading esterase (Est816) through rational design and its application in bioremediation - Fan_2023_Chemosphere_319_138021 |
| Author(s) : Fan X , Zhao M , Wen H , Zhang Y , Zhang J , Liu X |
| Ref : Chemosphere , 319 :138021 , 2023 |
| Abstract : Fan_2023_Chemosphere_319_138021 |
| ESTHER : Fan_2023_Chemosphere_319_138021 |
| PubMedSearch : Fan_2023_Chemosphere_319_138021 |
| PubMedID: 36731665 |
| Gene_locus related to this paper: 9bact-i6yrg4 |
| Title : A novel thermostable and salt-tolerant carboxylesterase involved in the initial aerobic degradation pathway for pyrethroids in Glycomyces salinus - Liu_2023_J.Hazard.Mater_451_131128 |
| Author(s) : Liu Y , Tang S , Wang X , Tang X , Wu Q , Huang Z , Ding J |
| Ref : J Hazard Mater , 451 :131128 , 2023 |
| Abstract : Liu_2023_J.Hazard.Mater_451_131128 |
| ESTHER : Liu_2023_J.Hazard.Mater_451_131128 |
| PubMedSearch : Liu_2023_J.Hazard.Mater_451_131128 |
| PubMedID: 36893599 |
| Gene_locus related to this paper: 9actn-EstGS1 |
| Title : Development and Application of a Life-Stage Physiologically Based Pharmacokinetic (PBPK) Model to the Assessment of Internal Dose of Pyrethroids in Humans - Mallick_2020_Toxicol.Sci_173_86 |
| Author(s) : Mallick P , Moreau M , Song G , Efremenko AY , Pendse SN , Creek MR , Osimitz TG , Hines RN , Hinderliter P , Clewell HJ , Lake BG , Yoon M |
| Ref : Toxicol Sci , 173 :86 , 2020 |
| Abstract : Mallick_2020_Toxicol.Sci_173_86 |
| ESTHER : Mallick_2020_Toxicol.Sci_173_86 |
| PubMedSearch : Mallick_2020_Toxicol.Sci_173_86 |
| PubMedID: 31593217 |
| Title : Metabolism of deltamethrin and cis- and trans-permethrin by rat and human liver microsomes, liver cytosol and plasma preparations - Hedges_2019_Xenobiotica_49_388 |
| Author(s) : Hedges L , Brown S , Vardy A , Doyle E , Yoon M , Osimitz TG , Lake BG |
| Ref : Xenobiotica , 49 :388 , 2019 |
| Abstract : Hedges_2019_Xenobiotica_49_388 |
| ESTHER : Hedges_2019_Xenobiotica_49_388 |
| PubMedSearch : Hedges_2019_Xenobiotica_49_388 |
| PubMedID: 29537356 |
| Title : Metabolism of deltamethrin and cis- and trans-permethrin by human expressed cytochrome P450 and carboxylesterase enzymes - Hedges_2019_Xenobiotica_49_521 |
| Author(s) : Hedges L , Brown S , MacLeod AK , Vardy A , Doyle E , Song G , Moreau M , Yoon M , Osimitz TG , Lake BG |
| Ref : Xenobiotica , 49 :521 , 2019 |
| Abstract : Hedges_2019_Xenobiotica_49_521 |
| ESTHER : Hedges_2019_Xenobiotica_49_521 |
| PubMedSearch : Hedges_2019_Xenobiotica_49_521 |
| PubMedID: 29779438 |
| Title : Esterase mediated resistance in deltamethrin resistant reference tick colony of Rhipicephalus (Boophilus) microplus - Gupta_2016_Exp.Appl.Acarol_69_239 |
| Author(s) : Gupta S , Ajith Kumar KG , Sharma AK , Nagar G , Kumar S , Saravanan BC , Ravikumar G , Ghosh S |
| Ref : Exp Appl Acarol , 69 :239 , 2016 |
| Abstract : Gupta_2016_Exp.Appl.Acarol_69_239 |
| ESTHER : Gupta_2016_Exp.Appl.Acarol_69_239 |
| PubMedSearch : Gupta_2016_Exp.Appl.Acarol_69_239 |
| PubMedID: 26979585 |
| Title : Testing the evolvability of an insect carboxylesterase for the detoxification of synthetic pyrethroid insecticides - Coppin_2012_Insect.Biochem.Mol.Biol_42_343 |
| Author(s) : Coppin CW , Jackson CJ , Sutherland T , Hart PJ , Devonshire AL , Russell RJ , Oakeshott JG |
| Ref : Insect Biochemistry & Molecular Biology , 42 :343 , 2012 |
| Abstract : Coppin_2012_Insect.Biochem.Mol.Biol_42_343 |
| ESTHER : Coppin_2012_Insect.Biochem.Mol.Biol_42_343 |
| PubMedSearch : Coppin_2012_Insect.Biochem.Mol.Biol_42_343 |
| PubMedID: 22300675 |
| Gene_locus related to this paper: luccu-E3aest7 |
| Title : The effect of pretreatment with S,S,S-tributyl phosphorotrithioate on deltamethrin resistance and carboxylesterase activity in Aphis gossypii (Glover) (Homoptera: Aphididae) - Chang_2010_Pestic.Biochem.Physiol_98_296 |
| Author(s) : Chang J , Cao C-W , Gao X-W |
| Ref : Pesticide Biochemistry and Physiology , 98 :296 , 2010 |
| Abstract : Chang_2010_Pestic.Biochem.Physiol_98_296 |
| ESTHER : Chang_2010_Pestic.Biochem.Physiol_98_296 |
| PubMedSearch : Chang_2010_Pestic.Biochem.Physiol_98_296 |
| PubMedID: |
| Title : Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin - Yang_2009_Biochem.Pharmacol_77_238 |
| Author(s) : Yang D , Pearce RE , Wang X , Gaedigk R , Wan YJ , Yan B |
| Ref : Biochemical Pharmacology , 77 :238 , 2009 |
| Abstract : Yang_2009_Biochem.Pharmacol_77_238 |
| ESTHER : Yang_2009_Biochem.Pharmacol_77_238 |
| PubMedSearch : Yang_2009_Biochem.Pharmacol_77_238 |
| PubMedID: 18983829 |
| Gene_locus related to this paper: human-CES1 , human-CES2 |
| Title : Is acetylcholinesterase a pertinent biomarker to detect exposure of pyrethroids? A study case with deltamethrin - Badiou_2008_Chem.Biol.Interact_175_406 |
| Author(s) : Badiou A , Belzunces LP |
| Ref : Chemico-Biological Interactions , 175 :406 , 2008 |
| Abstract : Badiou_2008_Chem.Biol.Interact_175_406 |
| ESTHER : Badiou_2008_Chem.Biol.Interact_175_406 |
| PubMedSearch : Badiou_2008_Chem.Biol.Interact_175_406 |
| PubMedID: 18602378 |
| Title : Honeybee Apis mellifera acetylcholinesterase--a biomarker to detect deltamethrin exposure - Badiou_2008_Ecotoxicol.Environ.Saf_69_246 |
| Author(s) : Badiou A , Meled M , Belzunces LP |
| Ref : Ecotoxicology & Environmental Safety , 69 :246 , 2008 |
| Abstract : Badiou_2008_Ecotoxicol.Environ.Saf_69_246 |
| ESTHER : Badiou_2008_Ecotoxicol.Environ.Saf_69_246 |
| PubMedSearch : Badiou_2008_Ecotoxicol.Environ.Saf_69_246 |
| PubMedID: 17215041 |
| Title : Species differences in the in vitro metabolism of deltamethrin and esfenvalerate: differential oxidative and hydrolytic metabolism by humans and rats - Godin_2006_Drug.Metab.Dispos_34_1764 |
| Author(s) : Godin SJ , Scollon EJ , Hughes MF , Potter PM , DeVito MJ , Ross MK |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 34 :1764 , 2006 |
| Abstract : Godin_2006_Drug.Metab.Dispos_34_1764 |
| ESTHER : Godin_2006_Drug.Metab.Dispos_34_1764 |
| PubMedSearch : Godin_2006_Drug.Metab.Dispos_34_1764 |
| PubMedID: 16855054 |
| Title : Cross-resistance to malathion in Cuban Culex quinquefasciatus induced by larval selection with deltamethrin - |
| Author(s) : Bisset J , Rodriguez M , Soca A |
| Ref : Med Vet Entomol , 12 :109 , 1998 |
| PubMedID: 9513948 |