DMCPE
General
Type : Cyclopropane || Alkyl ester
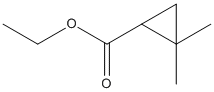
Chemical_Nomenclature : ethyl 2,2-dimethylcyclopropane-1-carboxylate
Canonical SMILES : CCOC(=O)C1CC1(C)C
InChI : InChI=1S\/C8H14O2\/c1-4-10-7(9)6-5-8(6,2)3\/h6H,4-5H2,1-3H3
InChIKey : OMQDMPAJKBUQSL-UHFFFAOYSA-N || OMQDMPAJKBUQSL-ZCFIWIBFSA-N || OMQDMPAJKBUQSL-LURJTMIESA-N
Other name(s) : SCHEMBL294716, Ethyl 2,2-dimethylcyclopropanecarboxylate, 2,2-Dimethyl-cyclopropanecarboxylic acid ethyl ester, Cyclopropanecarboxylic acid, 2,2-dimethyl-, ethyl ester
Target
Families : Haloperoxidase
References (2)
| Title : Structural and mechanistic insights into enantioselectivity toward near-symmetric esters of a novel carboxylesterase RoCE - Dou_2022_Catal.Sci.Technol_12_7448 |
| Author(s) : Dou Z , Jia P , Chen X , Wu Z , Xu G , Ni Y |
| Ref : Catal Sci Technol , 12 :7448 , 2022 |
| Abstract : Dou_2022_Catal.Sci.Technol_12_7448 |
| ESTHER : Dou_2022_Catal.Sci.Technol_12_7448 |
| PubMedSearch : Dou_2022_Catal.Sci.Technol_12_7448 |
| PubMedID: |
| Gene_locus related to this paper: rhoop-RoCE |
| Title : Enzymatic production of Cilastatin intermediate via highly enantioselective hydrolysis of methyl (+\/-)-2,2-dimethylcyclopropane carboxylate using newly isolated Rhodococcus sp. ECU1013 - Liu_2013_Appl.Microbiol.Biotechnol_97_7659 |
| Author(s) : Liu CH , Pan J , Ye Q , Xu JH |
| Ref : Applied Microbiology & Biotechnology , 97 :7659 , 2013 |
| Abstract : Liu_2013_Appl.Microbiol.Biotechnol_97_7659 |
| ESTHER : Liu_2013_Appl.Microbiol.Biotechnol_97_7659 |
| PubMedSearch : Liu_2013_Appl.Microbiol.Biotechnol_97_7659 |
| PubMedID: 23807665 |
| Gene_locus related to this paper: rhosp-AHY95170 |