DEHP
General
Type : Phthalate
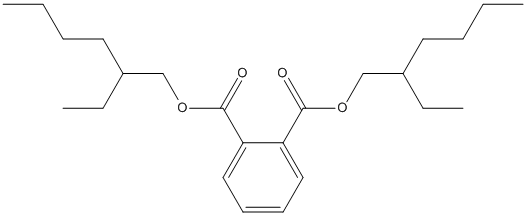
Chemical_Nomenclature : bis(2-ethylhexyl) benzene-1,2-dicarboxylate
Canonical SMILES : CCCCC(CC)COC(=O)C1=CC=CC=C1C(=O)OCC(CC)CCCC
InChI : InChI=1S\/C24H38O4\/c1-5-9-13-19(7-3)17-27-23(25)21-15-11-12-16-22(21)24(26)28-18-20(8-4)14-10-6-2\/h11-12,15-16,19-20H,5-10,13-14,17-18H2,1-4H3
InChIKey : BJQHLKABXJIVAM-UHFFFAOYSA-N
Other name(s) : Bis(2-ethylhexyl) phthalate, Di(2-ethylhexyl)phthalate, Di(2-ethylhexyl) phthalate, bis(2-ethylhexyl)phthalate, C6H4(COOC8H17)2
Target
References (17)
| Title : Biotoxicity responses of Zebrafish in environmentally relevant concentration of Di (2-ethylhexyl) phthalate - Li_2024_Environ.Toxicol.Pharmacol__104423 |
| Author(s) : Li X , Hu S , Jiang N , Yao X , Wang C , Wang Q , Yang Z , Wang J |
| Ref : Environ Toxicol Pharmacol , :104423 , 2024 |
| Abstract : Li_2024_Environ.Toxicol.Pharmacol__104423 |
| ESTHER : Li_2024_Environ.Toxicol.Pharmacol__104423 |
| PubMedSearch : Li_2024_Environ.Toxicol.Pharmacol__104423 |
| PubMedID: 38521434 |
| Title : Hydrolysis of dibutyl phthalate and di(2-ethylhexyl) phthalate in human liver, small intestine, kidney, and lung: An in vitro analysis using organ subcellular fractions and recombinant carboxylesterases - Isobe_2023_Chem.Biol.Interact_372_110353 |
| Author(s) : Isobe T , Ohkawara S , Mori Y , Jinno H , Tanaka-Kagawa T , Hanioka N |
| Ref : Chemico-Biological Interactions , 372 :110353 , 2023 |
| Abstract : Isobe_2023_Chem.Biol.Interact_372_110353 |
| ESTHER : Isobe_2023_Chem.Biol.Interact_372_110353 |
| PubMedSearch : Isobe_2023_Chem.Biol.Interact_372_110353 |
| PubMedID: 36657734 |
| Gene_locus related to this paper: human-CES1 , mouse-Ces1c |
| Title : Impacts of di-(2-ethylhexyl) phthalate on Folsomia candida (Collembola) assessed with a multi-biomarker approach - Zheng_2022_Ecotoxicol.Environ.Saf_232_113251 |
| Author(s) : Zheng Y , Zhou K , Tang J , Liu C , Bai J |
| Ref : Ecotoxicology & Environmental Safety , 232 :113251 , 2022 |
| Abstract : Zheng_2022_Ecotoxicol.Environ.Saf_232_113251 |
| ESTHER : Zheng_2022_Ecotoxicol.Environ.Saf_232_113251 |
| PubMedSearch : Zheng_2022_Ecotoxicol.Environ.Saf_232_113251 |
| PubMedID: 35121260 |
| Title : Multiple transcriptomic profiling: potential novel metabolism-related genes predict prepubertal testis damage caused by DEHP exposure - Kang_2021_Environ.Sci.Pollut.Res.Int__ |
| Author(s) : Kang L , Chen J , Wang J , Zhao T , Wei Y , Wu Y , Han L , Zheng X , Shen L , Long C , Wei G , Wu S |
| Ref : Environ Sci Pollut Res Int , : , 2021 |
| Abstract : Kang_2021_Environ.Sci.Pollut.Res.Int__ |
| ESTHER : Kang_2021_Environ.Sci.Pollut.Res.Int__ |
| PubMedSearch : Kang_2021_Environ.Sci.Pollut.Res.Int__ |
| PubMedID: 34595713 |
| Title : Biodegradation of Structurally Diverse Phthalate Esters by a Newly Identified Esterase with Catalytic Activity toward Di(2-ethylhexyl) Phthalate - Huang_2019_J.Agric.Food.Chem_67_8548 |
| Author(s) : Huang H , Zhang XY , Chen TL , Zhao YL , Xu DS , Bai YP |
| Ref : Journal of Agricultural and Food Chemistry , 67 :8548 , 2019 |
| Abstract : Huang_2019_J.Agric.Food.Chem_67_8548 |
| ESTHER : Huang_2019_J.Agric.Food.Chem_67_8548 |
| PubMedSearch : Huang_2019_J.Agric.Food.Chem_67_8548 |
| PubMedID: 31266305 |
| Gene_locus related to this paper: gorpv-h6n0g6 |
| Title : Biodegradation patterns of the endocrine disrupting pollutant di(2-ethyl hexyl) phthalate by Fusarium culmorum - Gonzalez-Marquez_2019_Ecotoxicol.Environ.Saf_170_293 |
| Author(s) : Gonzalez-Marquez A , Loera-Corral O , Santacruz-Juarez E , Tlecuitl-Beristain S , Garcia-Davila J , Viniegra-Gonzalez G , Sanchez C |
| Ref : Ecotoxicology & Environmental Safety , 170 :293 , 2019 |
| Abstract : Gonzalez-Marquez_2019_Ecotoxicol.Environ.Saf_170_293 |
| ESTHER : Gonzalez-Marquez_2019_Ecotoxicol.Environ.Saf_170_293 |
| PubMedSearch : Gonzalez-Marquez_2019_Ecotoxicol.Environ.Saf_170_293 |
| PubMedID: 30530181 |
| Title : Assessment of phthalate ester residues and distribution patterns in Baijiu raw materials and Baijiu - Dong_2019_Food.Chem_283_508 |
| Author(s) : Dong W , Guo R , Sun X , Li H , Zhao M , Zheng F , Sun J , Huang M , Wu J |
| Ref : Food Chem , 283 :508 , 2019 |
| Abstract : Dong_2019_Food.Chem_283_508 |
| ESTHER : Dong_2019_Food.Chem_283_508 |
| PubMedSearch : Dong_2019_Food.Chem_283_508 |
| PubMedID: 30722905 |
| Title : Di(2-ethylhexyl) phthalate induces apoptosis through mitochondrial pathway in GC-2spd cells - Fu_2017_Environ.Toxicol_32_1055 |
| Author(s) : Fu G , Dai J , Zhang D , Zhu L , Tang X , Zhang L , Zhou T , Duan P , Quan C , Zhang Z , Song S , Shi Y |
| Ref : Environ Toxicol , 32 :1055 , 2017 |
| Abstract : Fu_2017_Environ.Toxicol_32_1055 |
| ESTHER : Fu_2017_Environ.Toxicol_32_1055 |
| PubMedSearch : Fu_2017_Environ.Toxicol_32_1055 |
| PubMedID: 27416487 |
| Title : Re-characterization of mono-2-ethylhexyl phthalate hydrolase belonging to the serine hydrolase family - Iwata_2016_J.Biosci.Bioeng_122_140 |
| Author(s) : Iwata M , Imaoka T , Nishiyama T , Fujii T |
| Ref : J Biosci Bioeng , 122 :140 , 2016 |
| Abstract : Iwata_2016_J.Biosci.Bioeng_122_140 |
| ESTHER : Iwata_2016_J.Biosci.Bioeng_122_140 |
| PubMedSearch : Iwata_2016_J.Biosci.Bioeng_122_140 |
| PubMedID: 26868518 |
| Gene_locus related to this paper: 9noca-a0a0u5adh4 |
| Title : Enzymatic hydrolysis of structurally diverse phthalic acid esters by porcine and bovine pancreatic cholesterol esterases - Saito_2010_Chemosphere_81_1544 |
| Author(s) : Saito T , Hong P , Tanabe R , Nagai K , Kato K |
| Ref : Chemosphere , 81 :1544 , 2010 |
| Abstract : Saito_2010_Chemosphere_81_1544 |
| ESTHER : Saito_2010_Chemosphere_81_1544 |
| PubMedSearch : Saito_2010_Chemosphere_81_1544 |
| PubMedID: 20822795 |
| Gene_locus related to this paper: bovin-balip , pig-i3ltk9 |
| Title : Effect of di(2-ethylhexyl) phthalate (DEHP) on lipolysis and lipoprotein lipase activities in adipose tissue of rats - Martinelli_2010_Hum.Exp.Toxicol_29_739 |
| Author(s) : Martinelli MI , Mocchiutti NO , Bernal CA |
| Ref : Hum Exp Toxicol , 29 :739 , 2010 |
| Abstract : Martinelli_2010_Hum.Exp.Toxicol_29_739 |
| ESTHER : Martinelli_2010_Hum.Exp.Toxicol_29_739 |
| PubMedSearch : Martinelli_2010_Hum.Exp.Toxicol_29_739 |
| PubMedID: 20144957 |
| Title : A mono-2-ethylhexyl phthalate hydrolase from a Gordonia sp. that is able to dissimilate di-2-ethylhexyl phthalate - Nishioka_2006_Appl.Environ.Microbiol_72_2394 |
| Author(s) : Nishioka T , Iwata M , Imaoka T , Mutoh M , Egashira Y , Nishiyama T , Shin T , Fujii T |
| Ref : Applied Environmental Microbiology , 72 :2394 , 2006 |
| Abstract : Nishioka_2006_Appl.Environ.Microbiol_72_2394 |
| ESTHER : Nishioka_2006_Appl.Environ.Microbiol_72_2394 |
| PubMedSearch : Nishioka_2006_Appl.Environ.Microbiol_72_2394 |
| PubMedID: 16597936 |
| Gene_locus related to this paper: 9noca-a0a0u5adh4 , 9acto-q2mhh5 |
| Title : Species differences in the metabolism of di(2-ethylhexyl) phthalate (DEHP) in several organs of mice, rats, and marmosets - Ito_2005_Arch.Toxicol_79_147 |
| Author(s) : Ito Y , Yokota H , Wang R , Yamanoshita O , Ichihara G , Wang H , Kurata Y , Takagi K , Nakajima T |
| Ref : Archives of Toxicology , 79 :147 , 2005 |
| Abstract : Ito_2005_Arch.Toxicol_79_147 |
| ESTHER : Ito_2005_Arch.Toxicol_79_147 |
| PubMedSearch : Ito_2005_Arch.Toxicol_79_147 |
| PubMedID: 15798888 |
| Title : Degradation of an endocrine disrupting chemical, DEHP [di-(2-ethylhexyl)-phthalate], by Fusarium oxysporum f. sp. pisi cutinase - Kim_2003_Appl.Microbiol.Biotechnol_63_75 |
| Author(s) : Kim YH , Lee J , Moon SH |
| Ref : Applied Microbiology & Biotechnology , 63 :75 , 2003 |
| Abstract : Kim_2003_Appl.Microbiol.Biotechnol_63_75 |
| ESTHER : Kim_2003_Appl.Microbiol.Biotechnol_63_75 |
| PubMedSearch : Kim_2003_Appl.Microbiol.Biotechnol_63_75 |
| PubMedID: 12750855 |
| Gene_locus related to this paper: fusox-CUT |
| Title : The effect of the plasticizer di(2-ethylhexyl)phthalate on red cell deformability - Labow_1987_Blood_70_319 |
| Author(s) : Labow RS , Card RT , Rock G |
| Ref : Blood , 70 :319 , 1987 |
| Abstract : Labow_1987_Blood_70_319 |
| ESTHER : Labow_1987_Blood_70_319 |
| PubMedSearch : Labow_1987_Blood_70_319 |
| PubMedID: 3593970 |
| Title : Incorporation of plasticizer into red cells during storage - Rock_1984_Transfusion_24_493 |
| Author(s) : Rock G , Tocchi M , Ganz PR , Tackaberry ES |
| Ref : Transfusion , 24 :493 , 1984 |
| Abstract : Rock_1984_Transfusion_24_493 |
| ESTHER : Rock_1984_Transfusion_24_493 |
| PubMedSearch : Rock_1984_Transfusion_24_493 |
| PubMedID: 6506180 |
| Title : Metabolism of di-2-ethylhexyl phthalate by subcellular fractions from rainbow trout liver - Melancon_1977_Drug.Metab.Dispos_5_29 |
| Author(s) : Melancon MJ , Lech JJ |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 5 :29 , 1977 |
| Abstract : Melancon_1977_Drug.Metab.Dispos_5_29 |
| ESTHER : Melancon_1977_Drug.Metab.Dispos_5_29 |
| PubMedSearch : Melancon_1977_Drug.Metab.Dispos_5_29 |
| PubMedID: 13973 |