D-luciferin-methyl-ester
General
Type : Benzothiazol
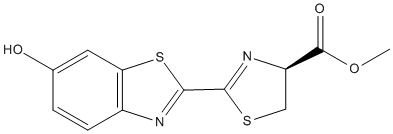
Chemical_Nomenclature : methyl (4S)-2-(6-hydroxy-1,3-benzothiazol-2-yl)-4,5-dihydro-1,3-thiazole-4-carboxylate
Canonical SMILES : COC(=O)C1CSC(=N1)C2=NC3=C(S2)C=C(C=C3)O
InChI : InChI=1S\/C12H10N2O3S2\/c1-17-12(16)8-5-18-10(14-8)11-13-7-3-2-6(15)4-9(7)19-11\/h2-4,8,15H,5H2,1H3\/t8-\/m1\/s1
InChIKey : PULFPBAWBRMDDC-MRVPVSSYSA-N
Other name(s) : DME, D-Luciferin,6'-Methyl Ether, D-Luciferin methyl, D-luciferin methyl ester, CHEMBL3427171, SCHEMBL12941013, ZINC149748264
MW : 294.34
Formula : C12H10N2O3S2
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Carb_B_Chordata
References (6)
| Title : Discovery of natural pentacyclic triterpenoids as potent and selective inhibitors against human carboxylesterase 1 - Song_2019_Fitoterapia_137_104199 |
| Author(s) : Song PF , Zhu YD , Ma HY , Wang YN , Wang DD , Zou LW , Ge GB , Yang L |
| Ref : Fitoterapia , 137 :104199 , 2019 |
| Abstract : Song_2019_Fitoterapia_137_104199 |
| ESTHER : Song_2019_Fitoterapia_137_104199 |
| PubMedSearch : Song_2019_Fitoterapia_137_104199 |
| PubMedID: 31175950 |
| Title : Nevadensin is a naturally occurring selective inhibitor of human carboxylesterase 1 - Wang_2018_Int.J.Biol.Macromol_120_1944 |
| Author(s) : Wang YQ , Weng ZM , Dou TY , Hou J , Wang DD , Ding LL , Zou LW , Yu Y , Chen J , Tang H , Ge GB |
| Ref : Int J Biol Macromol , 120 :1944 , 2018 |
| Abstract : Wang_2018_Int.J.Biol.Macromol_120_1944 |
| ESTHER : Wang_2018_Int.J.Biol.Macromol_120_1944 |
| PubMedSearch : Wang_2018_Int.J.Biol.Macromol_120_1944 |
| PubMedID: 30268757 |
| Title : Structure-Activity Relationships of Pentacyclic Triterpenoids as Potent and Selective Inhibitors against Human Carboxylesterase 1 - Zou_2017_Front.Pharmacol_8_435 |
| Author(s) : Zou LW , Dou TY , Wang P , Lei W , Weng ZM , Hou J , Wang DD , Fan YM , Zhang WD , Ge GB , Yang L |
| Ref : Front Pharmacol , 8 :435 , 2017 |
| Abstract : Zou_2017_Front.Pharmacol_8_435 |
| ESTHER : Zou_2017_Front.Pharmacol_8_435 |
| PubMedSearch : Zou_2017_Front.Pharmacol_8_435 |
| PubMedID: 28713276 |
| Title : Assessment of the inhibitory effects of pyrethroids against human carboxylesterases - Lei_2017_Toxicol.Appl.Pharmacol_321_48 |
| Author(s) : Lei W , Wang DD , Dou TY , Hou J , Feng L , Yin H , Luo Q , Sun J , Ge GB , Yang L |
| Ref : Toxicol Appl Pharmacol , 321 :48 , 2017 |
| Abstract : Lei_2017_Toxicol.Appl.Pharmacol_321_48 |
| ESTHER : Lei_2017_Toxicol.Appl.Pharmacol_321_48 |
| PubMedSearch : Lei_2017_Toxicol.Appl.Pharmacol_321_48 |
| PubMedID: 28242322 |
| Title : A new type of ultrasensitive bioluminogenic enzyme substrates. I. Enzyme substrates with D-luciferin as leaving group - Miska_1988_Biol.Chem.Hoppe.Seyler_369_407 |
| Author(s) : Miska W , Geiger R |
| Ref : Biol Chem Hoppe Seyler , 369 :407 , 1988 |
| Abstract : Miska_1988_Biol.Chem.Hoppe.Seyler_369_407 |
| ESTHER : Miska_1988_Biol.Chem.Hoppe.Seyler_369_407 |
| PubMedSearch : Miska_1988_Biol.Chem.Hoppe.Seyler_369_407 |
| PubMedID: 3166746 |
| Title : Synthesis and characterization of luciferin derivatives for use in bioluminescence enhanced enzyme immunoassays. New ultrasensitive detection systems for enzyme immunoassays, I - Miska_1987_J.Clin.Chem.Clin.Biochem_25_23 |
| Author(s) : Miska W , Geiger R |
| Ref : J Clinical Chemistry Clinical Biochemistry , 25 :23 , 1987 |
| Abstract : Miska_1987_J.Clin.Chem.Clin.Biochem_25_23 |
| ESTHER : Miska_1987_J.Clin.Chem.Clin.Biochem_25_23 |
| PubMedSearch : Miska_1987_J.Clin.Chem.Clin.Biochem_25_23 |
| PubMedID: 3549962 |