D-D-lactone
General
Type : Lactone || Ring opening polymerization substrate
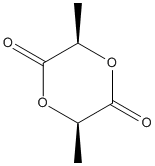
Chemical_Nomenclature : (3R,6R)-3,6-dimethyl-1,4-dioxane-2,5-dione
Canonical SMILES : CC1C(=O)OC(C(=O)O1)C
InChI : InChI=1S\/C6H8O4\/c1-3-5(7)10-4(2)6(8)9-3\/h3-4H,1-2H3\/t3-,4-\/m1\/s1
InChIKey : JJTUDXZGHPGLLC-QWWZWVQMSA-N
Other name(s) : d-lactide, (3R,6R)-3,6-dimethyl-1,4-dioxane-2,5-dione, 1,4-Dioxane-2,5-dione, 3,6-dimethyl-, (3R,6R)-, UNII-I1863O7V0Q, SCHEMBL682255, ZINC389773
Target
Families : Canar_LipB
References (3)
| Title : Enzymatic Ring-Opening Polymerization (ROP) of Polylactones: Roles of Non-Aqueous Solvents - Zhao_2018_J.Chem.Technol.Biotechnol_93_9 |
| Author(s) : Zhao H |
| Ref : J Chem Technol Biotechnol , 93 :9 , 2018 |
| Abstract : Zhao_2018_J.Chem.Technol.Biotechnol_93_9 |
| ESTHER : Zhao_2018_J.Chem.Technol.Biotechnol_93_9 |
| PubMedSearch : Zhao_2018_J.Chem.Technol.Biotechnol_93_9 |
| PubMedID: 31929672 |
| Title : Rational redesign of Candida antarctica lipase B for the ring opening polymerization of D,D-lactide - Takwa_2011_Chem.Commun.(Camb)_47_7392 |
| Author(s) : Takwa M , Larsen MW , Hult K , Martinelle M |
| Ref : Chem Commun (Camb) , 47 :7392 , 2011 |
| Abstract : Takwa_2011_Chem.Commun.(Camb)_47_7392 |
| ESTHER : Takwa_2011_Chem.Commun.(Camb)_47_7392 |
| PubMedSearch : Takwa_2011_Chem.Commun.(Camb)_47_7392 |
| PubMedID: 21607247 |
| Title : Branched poly(lactide) synthesized by enzymatic polymerization: effects of molecular branches and stereochemistry on enzymatic degradation and alkaline hydrolysis - Numata_2007_Biomacromolecules_8_3115 |
| Author(s) : Numata K , Srivastava RK , Finne-Wistrand A , Albertsson AC , Doi Y , Abe H |
| Ref : Biomacromolecules , 8 :3115 , 2007 |
| Abstract : Numata_2007_Biomacromolecules_8_3115 |
| ESTHER : Numata_2007_Biomacromolecules_8_3115 |
| PubMedSearch : Numata_2007_Biomacromolecules_8_3115 |
| PubMedID: 17722879 |