Cypermethrin
General
Type : pyrethroid || Insecticide || Phenoxyphenyl || Cyclopropane || Not A\/B H target || Cyanide
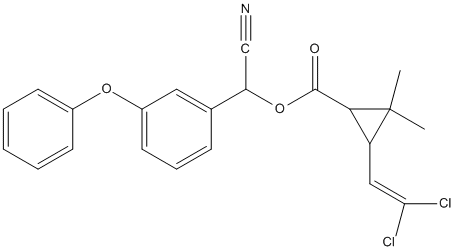
Chemical_Nomenclature : [cyano-(3-phenoxyphenyl)methyl] 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate
Canonical SMILES : CC1(C(C1C(=O)OC(C#N)C2=CC(=CC=C2)OC3=CC=CC=C3)C=C(Cl)Cl)C
InChI : InChI=1S\/C22H19Cl2NO3\/c1-22(2)17(12-19(23)24)20(22)21(26)28-18(13-25)14-7-6-10-16(11-14)27-15-8-4-3-5-9-15\/h3-12,17-18,20H,1-2H3
InChIKey : KAATUXNTWXVJKI-UHFFFAOYSA-N
Other name(s) : cypermethrine, Supercypermethrin, alpha-cypermethrin, Ripcord, Ammo, Beta-cypermethrin, Basathrin, Cymbush, Ustaad, Agrothrin, Creokhin, Cymperator, Cypercopal, Cyperkill, Cypersect, Flectron, Hilcyperin, Neramethrin, Polytrin, Arrivo, Cyperco, Kordon, Mustang, Sherpa, Siperin, Toppel, Ardap, Demon, Excis, Fenom, Colt, cis-Cypermethrin, Ambush C, Nrdc 149, Zeta-cypermethrin, SCHEMBL21972, CHEMBL373204, DB13721, CHEBI:4042, SCHEMBL5421473, SCHEMBL16766514, SCHEMBL8367208, SCHEMBL5322148
Target
References (19)
| Title : Enhancement degradation efficiency of pyrethroid-degrading esterase (Est816) through rational design and its application in bioremediation - Fan_2023_Chemosphere_319_138021 |
| Author(s) : Fan X , Zhao M , Wen H , Zhang Y , Zhang J , Liu X |
| Ref : Chemosphere , 319 :138021 , 2023 |
| Abstract : Fan_2023_Chemosphere_319_138021 |
| ESTHER : Fan_2023_Chemosphere_319_138021 |
| PubMedSearch : Fan_2023_Chemosphere_319_138021 |
| PubMedID: 36731665 |
| Gene_locus related to this paper: 9bact-i6yrg4 |
| Title : Resistance to Beta-cypermethrin, Azadirachtin, and Matrine, and Biochemical Characterization of Field Populations of Oedaleus asiaticus (Bey-Bienko) in Inner Mongolia, Northern China - Gao_2022_J.Insect.Sci_22_ |
| Author(s) : Gao S , Tan Y , Han H , Guo N , Gao H , Xu L , Lin K |
| Ref : J Insect Sci , 22 : , 2022 |
| Abstract : Gao_2022_J.Insect.Sci_22_ |
| ESTHER : Gao_2022_J.Insect.Sci_22_ |
| PubMedSearch : Gao_2022_J.Insect.Sci_22_ |
| PubMedID: 36374481 |
| Title : Overexpression of PxalphaE14 Contributing to Detoxification of Multiple Insecticides in Plutella xylostella (L.) - Li_2022_J.Agric.Food.Chem_70_5794 |
| Author(s) : Li R , Zhu B , Hu XP , Shi XY , Qi LL , Liang P , Gao XW |
| Ref : Journal of Agricultural and Food Chemistry , 70 :5794 , 2022 |
| Abstract : Li_2022_J.Agric.Food.Chem_70_5794 |
| ESTHER : Li_2022_J.Agric.Food.Chem_70_5794 |
| PubMedSearch : Li_2022_J.Agric.Food.Chem_70_5794 |
| PubMedID: 35510781 |
| Gene_locus related to this paper: pluxy-PxalphaE14 |
| Title : Two single mutations in carboxylesterase 001C improve fenvalerate hydrolase activity in Helicoverpa armigera - Xu_2021_Pestic.Biochem.Physiol_179_104969 |
| Author(s) : Xu JJ , Chang YM , Lu M , Tie Y , Dong YL , Chen GY , Ma ZQ , Liu XL , Li YQ |
| Ref : Pestic Biochem Physiol , 179 :104969 , 2021 |
| Abstract : Xu_2021_Pestic.Biochem.Physiol_179_104969 |
| ESTHER : Xu_2021_Pestic.Biochem.Physiol_179_104969 |
| PubMedSearch : Xu_2021_Pestic.Biochem.Physiol_179_104969 |
| PubMedID: 34802519 |
| Gene_locus related to this paper: helam-d5kx87 |
| Title : Enhanced hydrolysis of beta-cypermethrin caused by deletions in the glycin-rich region of carboxylesterase 001G from Helicoverpa armigera - Bai_2021_Pest.Manag.Sci_77_2129 |
| Author(s) : Bai LS , Xu JJ , Zhao CX , Chang YL , Dong YL , Zhang KG , Li YQ , Li YP , Ma ZQ , Liu XL |
| Ref : Pest Manag Sci , 77 :2129 , 2021 |
| Abstract : Bai_2021_Pest.Manag.Sci_77_2129 |
| ESTHER : Bai_2021_Pest.Manag.Sci_77_2129 |
| PubMedSearch : Bai_2021_Pest.Manag.Sci_77_2129 |
| PubMedID: 33336552 |
| Gene_locus related to this paper: helam-d5g3d4 |
| Title : Functional analysis of a carboxylesterase gene involved in beta-cypermethrin and phoxim resistance in Plutella xylostella (L.) - Li_2020_Pest.Manag.Sci_77_2097 |
| Author(s) : Li R , Zhu B , Shan J , Li L , Liang P , Gao X |
| Ref : Pest Manag Sci , 77\ :2097 , 2020 |
| Abstract : Li_2020_Pest.Manag.Sci_77_2097 |
| ESTHER : Li_2020_Pest.Manag.Sci_77_2097 |
| PubMedSearch : Li_2020_Pest.Manag.Sci_77_2097 |
| PubMedID: 33342080 |
| Gene_locus related to this paper: pluxy-PxalphaE8 |
| Title : Identification of key residues of carboxylesterase PxEst-6 involved in pyrethroid metabolism in Plutella xylostella (L.) - Li_2020_J.Hazard.Mater_407_124612 |
| Author(s) : Li Y , Sun H , Tian Z , Ye X , Li R , Li X , Zheng S , Liu J , Zhang Y |
| Ref : J Hazard Mater , 407 :124612 , 2020 |
| Abstract : Li_2020_J.Hazard.Mater_407_124612 |
| ESTHER : Li_2020_J.Hazard.Mater_407_124612 |
| PubMedSearch : Li_2020_J.Hazard.Mater_407_124612 |
| PubMedID: 33338816 |
| Gene_locus related to this paper: pluxy-f1c4b8 |
| Title : Functional Characterization of two Carboxylesterase Genes Involved in Pyrethroid Detoxification in Helicoverpa armigera - Li_2020_J.Agric.Food.Chem_68_3390 |
| Author(s) : Li Y , Bai L , Zhao C , Xu J , Sun Z , Dong Y , Li D , Liu XL , Ma ZQ |
| Ref : Journal of Agricultural and Food Chemistry , 68 :3390 , 2020 |
| Abstract : Li_2020_J.Agric.Food.Chem_68_3390 |
| ESTHER : Li_2020_J.Agric.Food.Chem_68_3390 |
| PubMedSearch : Li_2020_J.Agric.Food.Chem_68_3390 |
| PubMedID: 32096985 |
| Gene_locus related to this paper: helam-d5g3d5 , helam-d9iv61 |
| Title : Monitoring and biochemical characterization of beta-cypermethrin resistance in Spodoptera exigua (Lepidoptera: Noctuidae) in Sichuan Province, China - Wang_2018_Pestic.Biochem.Physiol_146_71 |
| Author(s) : Wang X , Xiang X , Yu H , Liu S , Yin Y , Cui P , Wu Y , Yang J , Jiang C , Yang Q |
| Ref : Pestic Biochem Physiol , 146 :71 , 2018 |
| Abstract : Wang_2018_Pestic.Biochem.Physiol_146_71 |
| ESTHER : Wang_2018_Pestic.Biochem.Physiol_146_71 |
| PubMedSearch : Wang_2018_Pestic.Biochem.Physiol_146_71 |
| PubMedID: 29626995 |
| Title : Determination of cypermethrin degradation potential of soil bacteria along with plant growth-promoting characteristics - Akbar_2015_Curr.Microbiol_70_75 |
| Author(s) : Akbar S , Sultan S , Kertesz M |
| Ref : Curr Microbiol , 70 :75 , 2015 |
| Abstract : Akbar_2015_Curr.Microbiol_70_75 |
| ESTHER : Akbar_2015_Curr.Microbiol_70_75 |
| PubMedSearch : Akbar_2015_Curr.Microbiol_70_75 |
| PubMedID: 25194282 |
| Title : Organophosphate and pyrethroid hydrolase activities of mutant Esterases from the cotton bollworm Helicoverpa armigera - Li_2013_PLoS.One_8_e77685 |
| Author(s) : Li Y , Farnsworth CA , Coppin CW , Teese MG , Liu JW , Scott C , Zhang X , Russell RJ , Oakeshott JG |
| Ref : PLoS ONE , 8 :e77685 , 2013 |
| Abstract : Li_2013_PLoS.One_8_e77685 |
| ESTHER : Li_2013_PLoS.One_8_e77685 |
| PubMedSearch : Li_2013_PLoS.One_8_e77685 |
| PubMedID: 24204917 |
| Gene_locus related to this paper: helam-d5g3c9 , helam-d5g3d2 , helam-d5g3d3 , helam-d5g3d4 , helam-d5g3d5 , helam-d5kx87 , helam-d5kx99 , helam-d5kxa9 , helam-d9iv61 , helam-d9iv62 , helam-h9zvh4 , helam-s4wfz6 |
| Title : Molecular cloning, purification and biochemical characterization of a novel pyrethroid-hydrolyzing carboxylesterase gene from Ochrobactrum anthropi YZ-1 - Zhai_2012_J.Hazard.Mater_221-222_206 |
| Author(s) : Zhai Y , Li K , Song J , Shi Y , Yan Y |
| Ref : J Hazard Mater , 221-222 :206 , 2012 |
| Abstract : Zhai_2012_J.Hazard.Mater_221-222_206 |
| ESTHER : Zhai_2012_J.Hazard.Mater_221-222_206 |
| PubMedSearch : Zhai_2012_J.Hazard.Mater_221-222_206 |
| PubMedID: 22579404 |
| Gene_locus related to this paper: 9hyph-h2esq9 |
| Title : Quantitative and qualitative changes of the carboxylesterase associated with beta-cypermethrin resistance in the housefly, Musca domestica (Diptera: Muscidae) - Zhang_2010_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_156_6 |
| Author(s) : Zhang L , Shi J , Shi X , Liang P , Gao J , Gao X |
| Ref : Comparative Biochemistry & Physiology B Biochem Mol Biol , 156 :6 , 2010 |
| Abstract : Zhang_2010_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_156_6 |
| ESTHER : Zhang_2010_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_156_6 |
| PubMedSearch : Zhang_2010_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_156_6 |
| PubMedID: 20117228 |
| Gene_locus related to this paper: musdo-EST23aes07 |
| Title : Hydrolysis of individual isomers of fluorogenic pyrethroid analogs by mutant carboxylesterases from Lucilia cuprina - Devonshire_2007_Insect.Biochem.Mol.Biol_37_891 |
| Author(s) : Devonshire AL , Heidari R , Huang HZ , Hammock BD , Russell RJ , Oakeshott JG |
| Ref : Insect Biochemistry & Molecular Biology , 37 :891 , 2007 |
| Abstract : Devonshire_2007_Insect.Biochem.Mol.Biol_37_891 |
| ESTHER : Devonshire_2007_Insect.Biochem.Mol.Biol_37_891 |
| PubMedSearch : Devonshire_2007_Insect.Biochem.Mol.Biol_37_891 |
| PubMedID: 17681228 |
| Gene_locus related to this paper: luccu-E3aest7 |
| Title : Beta-cypermethrin resistance associated with high carboxylesterase activities in a strain of house fly, Musca domestica (Diptera: Muscidae) - Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| Author(s) : Zhang L , Gao X , Liang P |
| Ref : Pesticide Biochemistry and Physiology , 89 :65 , 2007 |
| Abstract : Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| ESTHER : Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| PubMedSearch : Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| PubMedID: |
| Title : Characterization of pyrethroid hydrolysis by the human liver carboxylesterases hCE-1 and hCE-2 - Nishi_2006_Arch.Biochem.Biophys_445_115 |
| Author(s) : Nishi K , Huang H , Kamita SG , Kim IH , Morisseau C , Hammock BD |
| Ref : Archives of Biochemistry & Biophysics , 445 :115 , 2006 |
| Abstract : Nishi_2006_Arch.Biochem.Biophys_445_115 |
| ESTHER : Nishi_2006_Arch.Biochem.Biophys_445_115 |
| PubMedSearch : Nishi_2006_Arch.Biochem.Biophys_445_115 |
| PubMedID: 16359636 |
| Gene_locus related to this paper: human-CES1 , human-CES2 |
| Title : Identification, expression, and purification of a pyrethroid-hydrolyzing carboxylesterase from mouse liver microsomes - Stok_2004_J.Biol.Chem_279_29863 |
| Author(s) : Stok JE , Huang H , Jones PD , Wheelock CE , Morisseau C , Hammock BD |
| Ref : Journal of Biological Chemistry , 279 :29863 , 2004 |
| Abstract : Stok_2004_J.Biol.Chem_279_29863 |
| ESTHER : Stok_2004_J.Biol.Chem_279_29863 |
| PubMedSearch : Stok_2004_J.Biol.Chem_279_29863 |
| PubMedID: 15123619 |
| Gene_locus related to this paper: mouse-Ces2a , mouse-Ces2e |
| Title : A Microsomal Esterase Involved in Cypermethrin Resistance in the German Cockroach, Blattella germanica - Valles_2001_Pestic.Biochem.Physiol_71_56 |
| Author(s) : Valles SM , Strong CA |
| Ref : Pesticide Biochemistry and Physiology , 71 :56 , 2001 |
| Abstract : Valles_2001_Pestic.Biochem.Physiol_71_56 |
| ESTHER : Valles_2001_Pestic.Biochem.Physiol_71_56 |
| PubMedSearch : Valles_2001_Pestic.Biochem.Physiol_71_56 |
| PubMedID: |
| Title : Clinico-biochemical use of serum acetylcholine esterase following treatment with synthetic pyrethroids, cypermethrin and fenvalerate, in cattle and buffalo experimentally infested with Boophilus microplus - Ansari_1990_Indian.J.Exp.Biol_28_241 |
| Author(s) : Ansari MZ , Kumar A , Prasad RL , Basu A , Sahai BN , Sinha AP |
| Ref : Indian J Exp Biol , 28 :241 , 1990 |
| Abstract : Ansari_1990_Indian.J.Exp.Biol_28_241 |
| ESTHER : Ansari_1990_Indian.J.Exp.Biol_28_241 |
| PubMedSearch : Ansari_1990_Indian.J.Exp.Biol_28_241 |
| PubMedID: 2365420 |