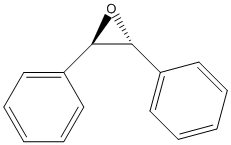
Cis-stilbene-oxide
General
Type : Epoxide
Chemical_Nomenclature : (2R,3S)-2,3-diphenyloxirane
Canonical SMILES : C1=CC=C(C=C1)C2C(O2)C3=CC=CC=C3
InChI : InChI=1S\/C14H12O\/c1-3-7-11(8-4-1)13-14(15-13)12-9-5-2-6-10-12\/h1-10,13-14H\/t13-,14+
InChIKey : ARCJQKUWGAZPFX-OKILXGFUSA-N
Other name(s) : Cis-Stilbene oxide, (2R,3S)-2,3-diphenyloxirane, UNII-GIX80CN6Z7, 1689-71-0, CCRIS 2080, Trans-Stilbene oxide, cSO
MW : 196.24
Formula : C14H12O
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : CFTR-inhibitory-factor_Cif, Epoxide_hydrolase
References (11)
| Title : Active-Site Flexibility and Substrate Specificity in a Bacterial Virulence Factor: Crystallographic Snapshots of an Epoxide Hydrolase - Hvorecny_2017_Structure_25_697 |
| Author(s) : Hvorecny KL , Bahl CD , Kitamura S , Lee KSS , Hammock BD , Morisseau C , Madden DR |
| Ref : Structure , 25 :697 , 2017 |
| Abstract : Hvorecny_2017_Structure_25_697 |
| ESTHER : Hvorecny_2017_Structure_25_697 |
| PubMedSearch : Hvorecny_2017_Structure_25_697 |
| PubMedID: 28392259 |
| Gene_locus related to this paper: pseae-PA2934 |
| Title : Development of fluorescent substrates for microsomal epoxide hydrolase and application to inhibition studies - Morisseau_2011_Anal.Biochem_414_154 |
| Author(s) : Morisseau C , Bernay M , Escaich A , Sanborn JR , Lango J , Hammock BD |
| Ref : Analytical Biochemistry , 414 :154 , 2011 |
| Abstract : Morisseau_2011_Anal.Biochem_414_154 |
| ESTHER : Morisseau_2011_Anal.Biochem_414_154 |
| PubMedSearch : Morisseau_2011_Anal.Biochem_414_154 |
| PubMedID: 21371418 |
| Gene_locus related to this paper: human-EPHX1 , ratno-hyep |
| Title : Development of metabolically stable inhibitors of Mammalian microsomal epoxide hydrolase - Morisseau_2008_Chem.Res.Toxicol_21_951 |
| Author(s) : Morisseau C , Newman JW , Wheelock CE , Hill Iii T , Morin D , Buckpitt AR , Hammock BD |
| Ref : Chemical Research in Toxicology , 21 :951 , 2008 |
| Abstract : Morisseau_2008_Chem.Res.Toxicol_21_951 |
| ESTHER : Morisseau_2008_Chem.Res.Toxicol_21_951 |
| PubMedSearch : Morisseau_2008_Chem.Res.Toxicol_21_951 |
| PubMedID: 18363382 |
| Gene_locus related to this paper: human-EPHX1 , ratno-hyep |
| Title : Regulation of JH epoxide hydrolase versus JH esterase activity in the cabbage looper, Trichoplusia ni, by juvenile hormone and xenobiotics - Anspaugh_2005_J.Insect.Physiol_51_523 |
| Author(s) : Anspaugh DD , Roe RM |
| Ref : J Insect Physiol , 51 :523 , 2005 |
| Abstract : Anspaugh_2005_J.Insect.Physiol_51_523 |
| ESTHER : Anspaugh_2005_J.Insect.Physiol_51_523 |
| PubMedSearch : Anspaugh_2005_J.Insect.Physiol_51_523 |
| PubMedID: 15893999 |
| Title : The Saccharomyces cerevisiae ORF YNR064c protein has characteristics of an 'orphaned' epoxide hydrolase - Elfstrom_2005_Biochim.Biophys.Acta_1748_213 |
| Author(s) : Elfstrom LT , Widersten M |
| Ref : Biochimica & Biophysica Acta , 1748 :213 , 2005 |
| Abstract : Elfstrom_2005_Biochim.Biophys.Acta_1748_213 |
| ESTHER : Elfstrom_2005_Biochim.Biophys.Acta_1748_213 |
| PubMedSearch : Elfstrom_2005_Biochim.Biophys.Acta_1748_213 |
| PubMedID: 15769598 |
| Gene_locus related to this paper: yeast-SCYNR064C |
| Title : Functional analysis of human microsomal epoxide hydrolase genetic variants - Hosagrahara_2004_Chem.Biol.Interact_150_149 |
| Author(s) : Hosagrahara VP , Rettie AE , Hassett C , Omiecinski CJ |
| Ref : Chemico-Biological Interactions , 150 :149 , 2004 |
| Abstract : Hosagrahara_2004_Chem.Biol.Interact_150_149 |
| ESTHER : Hosagrahara_2004_Chem.Biol.Interact_150_149 |
| PubMedSearch : Hosagrahara_2004_Chem.Biol.Interact_150_149 |
| PubMedID: 15535985 |
| Gene_locus related to this paper: human-EPHX1 |
| Title : Characterization and cDNA cloning of a clofibrate-inducible microsomal epoxide hydrolase in Drosophila melanogaster - Taniai_2003_Eur.J.Biochem_270_4696 |
| Author(s) : Taniai K , Inceoglu AB , Yukuhiro K , Hammock BD |
| Ref : European Journal of Biochemistry , 270 :4696 , 2003 |
| Abstract : Taniai_2003_Eur.J.Biochem_270_4696 |
| ESTHER : Taniai_2003_Eur.J.Biochem_270_4696 |
| PubMedSearch : Taniai_2003_Eur.J.Biochem_270_4696 |
| PubMedID: 14622257 |
| Gene_locus related to this paper: drome-CG15102 |
| Title : Expression and activity of microsomal epoxide hydrolase in follicles isolated from mouse ovaries - Cannady_2002_Toxicol.Sci_68_24 |
| Author(s) : Cannady EA , Dyer CA , Christian PJ , Sipes IG , Hoyer PB |
| Ref : Toxicol Sci , 68 :24 , 2002 |
| Abstract : Cannady_2002_Toxicol.Sci_68_24 |
| ESTHER : Cannady_2002_Toxicol.Sci_68_24 |
| PubMedSearch : Cannady_2002_Toxicol.Sci_68_24 |
| PubMedID: 12075107 |
| Gene_locus related to this paper: mouse-EPHX1 |
| Title : Overexpression of Arabidopsis thaliana soluble epoxide hydrolase 1 in Pichia pastoris and characterisation of the recombinant enzyme - Bellevik_2002_Protein.Expr.Purif_26_65 |
| Author(s) : Bellevik S , Summerer S , Meijer J |
| Ref : Protein Expr Purif , 26 :65 , 2002 |
| Abstract : Bellevik_2002_Protein.Expr.Purif_26_65 |
| ESTHER : Bellevik_2002_Protein.Expr.Purif_26_65 |
| PubMedSearch : Bellevik_2002_Protein.Expr.Purif_26_65 |
| PubMedID: 12356472 |
| Gene_locus related to this paper: arath-At2g26740 |
| Title : Interindividual and interspecies variation in hepatic microsomal epoxide hydrolase activity: studies with cis-stilbene oxide, carbamazepine 10, 11-epoxide and naphthalene - Kitteringham_1996_J.Pharmacol.Exp.Ther_278_1018 |
| Author(s) : Kitteringham NR , Davis C , Howard N , Pirmohamed M , Park BK |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 278 :1018 , 1996 |
| Abstract : Kitteringham_1996_J.Pharmacol.Exp.Ther_278_1018 |
| ESTHER : Kitteringham_1996_J.Pharmacol.Exp.Ther_278_1018 |
| PubMedSearch : Kitteringham_1996_J.Pharmacol.Exp.Ther_278_1018 |
| PubMedID: 8819481 |
| Title : The effect of tridiphane (2-(3,5-dichlorophenyl)-2-(2,2,2-trichloroethyl)oxirane) on hepatic epoxide-metabolizing enzymes: indications of peroxisome proliferation - Moody_1987_Toxicol.Appl.Pharmacol_89_37 |
| Author(s) : Moody DE , Hammock BD |
| Ref : Toxicol Appl Pharmacol , 89 :37 , 1987 |
| Abstract : Moody_1987_Toxicol.Appl.Pharmacol_89_37 |
| ESTHER : Moody_1987_Toxicol.Appl.Pharmacol_89_37 |
| PubMedSearch : Moody_1987_Toxicol.Appl.Pharmacol_89_37 |
| PubMedID: 3590187 |