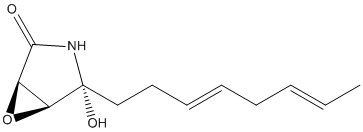
Cerulenin-hemiaminal
General
Type : Epoxide || Azabicyclo
Chemical_Nomenclature : (1R,4R,5S)-4-hydroxy-4-[(3E,6E)-octa-3,6-dienyl]-6-oxa-3-azabicyclo[3.1.0]hexan-2-one
Canonical SMILES : CC=CCC=CCCC1(C2C(O2)C(=O)N1)O
InChI : InChI=1S\/C12H17NO3\/c1-2-3-4-5-6-7-8-12(15)10-9(16-10)11(14)13-12\/h2-3,5-6,9-10,15H,4,7-8H2,1H3,(H,13,14)\/b3-2+,6-5+\/t9-,10+,12-\/m1\/s1
InChIKey : XYRMBRBAWFZQDU-NUBWZSAQSA-N
Other name(s) : SCHEMBL12202400
MW : 223.27
Formula : C12H17NO3
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Dieckmann_Cyclase
References (1)
| Title : Structural Basis for Enzymatic Off-Loading of Hybrid Polyketides by Dieckmann Condensation - Cogan_2020_ACS.Chem.Biol_15_2783 |
| Author(s) : Cogan DP , Ly J , Nair SK |
| Ref : ACS Chemical Biology , 15 :2783 , 2020 |
| Abstract : Cogan_2020_ACS.Chem.Biol_15_2783 |
| ESTHER : Cogan_2020_ACS.Chem.Biol_15_2783 |
| PubMedSearch : Cogan_2020_ACS.Chem.Biol_15_2783 |
| PubMedID: 33017142 |
| Gene_locus related to this paper: 9pseu-NcmC |