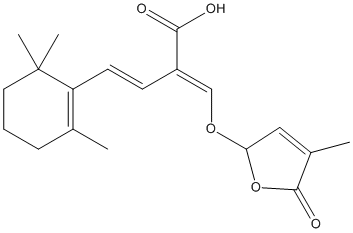
Carlactonoic-acid
General
Type : Butenolide || Strigolactone receptors ligand || Non-canonical strigolactone
Chemical_Nomenclature : (E,2E)-2-[(4-methyl-5-oxo-2H-furan-2-yl)oxymethylidene]-4-(2,6,6-trimethylcyclohexen-1-yl)but-3-enoic acid
Canonical SMILES : CC1=C(C(CCC1)(C)C)C=CC(=COC2C=C(C(=O)O2)C)C(=O)O
InChI : InChI=1S\/C19H24O5\/c1-12-6-5-9-19(3,4)15(12)8-7-14(17(20)21)11-23-16-10-13(2)18(22)24-16\/h7-8,10-11,16H,5-6,9H2,1-4H3,(H,20,21)\/b8-7+,14-11+
InChIKey : WUBRWXCVIPHXLX-DQBULIGTSA-N
Other name(s) : Carlactonoic acid, J3.622.201H, Carlactonoate, CLA
MW : 332.4
Formula : C19H24O5
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : RsbQ-like
References (13)
| Title : Conversion of methyl carlactonoate to heliolactone in sunflower - Wakabayashi_2022_Nat.Prod.Res_36_2215 |
| Author(s) : Wakabayashi T , Shinde H , Shiotani N , Yamamoto S , Mizutani M , Takikawa H , Sugimoto Y |
| Ref : Nat Prod Res , 36 :2215 , 2022 |
| Abstract : Wakabayashi_2022_Nat.Prod.Res_36_2215 |
| ESTHER : Wakabayashi_2022_Nat.Prod.Res_36_2215 |
| PubMedSearch : Wakabayashi_2022_Nat.Prod.Res_36_2215 |
| PubMedID: 33034235 |
| Title : Hydroxyl carlactone derivatives are predominant strigolactones in Arabidopsis - Yoneyama_2020_Plant.Direct_4_e00219 |
| Author(s) : Yoneyama K , Akiyama K , Brewer PB , Mori N , Kawano-Kawada M , Haruta S , Nishiwaki H , Yamauchi S , Xie X , Umehara M , Beveridge CA , Nomura T |
| Ref : Plant Direct , 4 :e00219 , 2020 |
| Abstract : Yoneyama_2020_Plant.Direct_4_e00219 |
| ESTHER : Yoneyama_2020_Plant.Direct_4_e00219 |
| PubMedSearch : Yoneyama_2020_Plant.Direct_4_e00219 |
| PubMedID: 32399509 |
| Title : An improved strategy to analyse strigolactones in complex sample matrices using UHPLC-MS\/MS - Flokova_2020_Plant.Methods_16_125 |
| Author(s) : Flokova K , Shimels M , Andreo Jimenez B , Bardaro N , Strnad M , Novak O , Bouwmeester HJ |
| Ref : Plant Methods , 16 :125 , 2020 |
| Abstract : Flokova_2020_Plant.Methods_16_125 |
| ESTHER : Flokova_2020_Plant.Methods_16_125 |
| PubMedSearch : Flokova_2020_Plant.Methods_16_125 |
| PubMedID: 32963580 |
| Title : Chemical identification of 18-hydroxycarlactonoic acid as an LjMAX1 product and in planta conversion of its methyl ester to canonical and non-canonical strigolactones in Lotus japonicus - Mori_2020_Phytochemistry_174_112349 |
| Author(s) : Mori N , Sado A , Xie X , Yoneyama K , Asami K , Seto Y , Nomura T , Yamaguchi S , Akiyama K |
| Ref : Phytochemistry , 174 :112349 , 2020 |
| Abstract : Mori_2020_Phytochemistry_174_112349 |
| ESTHER : Mori_2020_Phytochemistry_174_112349 |
| PubMedSearch : Mori_2020_Phytochemistry_174_112349 |
| PubMedID: 32213359 |
| Title : Direct conversion of carlactonoic acid to orobanchol by cytochrome P450 CYP722C in strigolactone biosynthesis - Wakabayashi_2019_Sci.Adv_5_eaax9067 |
| Author(s) : Wakabayashi T , Hamana M , Mori A , Akiyama R , Ueno K , Osakabe K , Osakabe Y , Suzuki H , Takikawa H , Mizutani M , Sugimoto Y |
| Ref : Sci Adv , 5 :eaax9067 , 2019 |
| Abstract : Wakabayashi_2019_Sci.Adv_5_eaax9067 |
| ESTHER : Wakabayashi_2019_Sci.Adv_5_eaax9067 |
| PubMedSearch : Wakabayashi_2019_Sci.Adv_5_eaax9067 |
| PubMedID: 32064317 |
| Title : The tomato MAX1 homolog, SlMAX1, is involved in the biosynthesis of tomato strigolactones from carlactone - Zhang_2018_New.Phytol_219_297 |
| Author(s) : Zhang Y , Cheng X , Wang Y , Diez-Simon C , Flokova K , Bimbo A , Bouwmeester HJ , Ruyter-Spira C |
| Ref : New Phytol , 219 :297 , 2018 |
| Abstract : Zhang_2018_New.Phytol_219_297 |
| ESTHER : Zhang_2018_New.Phytol_219_297 |
| PubMedSearch : Zhang_2018_New.Phytol_219_297 |
| PubMedID: 29655242 |
| Title : Evidence for species-dependent biosynthetic pathways for converting carlactone to strigolactones in plants - Iseki_2018_J.Exp.Bot_69_2305 |
| Author(s) : Iseki M , Shida K , Kuwabara K , Wakabayashi T , Mizutani M , Takikawa H , Sugimoto Y |
| Ref : J Exp Bot , 69 :2305 , 2018 |
| Abstract : Iseki_2018_J.Exp.Bot_69_2305 |
| ESTHER : Iseki_2018_J.Exp.Bot_69_2305 |
| PubMedSearch : Iseki_2018_J.Exp.Bot_69_2305 |
| PubMedID: 29294064 |
| Title : Conversion of carlactone to carlactonoic acid is a conserved function of MAX1 homologs in strigolactone biosynthesis - Yoneyama_2018_New.Phytol_218_1522 |
| Author(s) : Yoneyama K , Mori N , Sato T , Yoda A , Xie X , Okamoto M , Iwanaga M , Ohnishi T , Nishiwaki H , Asami T , Yokota T , Akiyama K , Nomura T |
| Ref : New Phytol , 218 :1522 , 2018 |
| Abstract : Yoneyama_2018_New.Phytol_218_1522 |
| ESTHER : Yoneyama_2018_New.Phytol_218_1522 |
| PubMedSearch : Yoneyama_2018_New.Phytol_218_1522 |
| PubMedID: 29479714 |
| Title : Which are the major players, canonical or non-canonical strigolactones? - Yoneyama_2018_J.Exp.Bot_69_2231 |
| Author(s) : Yoneyama K , Xie X , Kisugi T , Nomura T , Nakatani Y , Akiyama K , McErlean CSP |
| Ref : J Exp Bot , 69 :2231 , 2018 |
| Abstract : Yoneyama_2018_J.Exp.Bot_69_2231 |
| ESTHER : Yoneyama_2018_J.Exp.Bot_69_2231 |
| PubMedSearch : Yoneyama_2018_J.Exp.Bot_69_2231 |
| PubMedID: 29522151 |
| Title : Synthetic Access to Noncanonical Strigolactones: Syntheses of Carlactonic Acid and Methyl Carlactonoate - Dieckmann_2018_J.Org.Chem_83_125 |
| Author(s) : Dieckmann MC , Dakas PY , De Mesmaeker A |
| Ref : J Org Chem , 83 :125 , 2018 |
| Abstract : Dieckmann_2018_J.Org.Chem_83_125 |
| ESTHER : Dieckmann_2018_J.Org.Chem_83_125 |
| PubMedSearch : Dieckmann_2018_J.Org.Chem_83_125 |
| PubMedID: 29179551 |
| Title : Zealactones. Novel natural strigolactones from maize - Charnikhova_2017_Phytochemistry_137_123 |
| Author(s) : Charnikhova TV , Gaus K , Lumbroso A , Sanders M , Vincken JP , De Mesmaeker A , Ruyter-Spira CP , Screpanti C , Bouwmeester HJ |
| Ref : Phytochemistry , 137 :123 , 2017 |
| Abstract : Charnikhova_2017_Phytochemistry_137_123 |
| ESTHER : Charnikhova_2017_Phytochemistry_137_123 |
| PubMedSearch : Charnikhova_2017_Phytochemistry_137_123 |
| PubMedID: 28215609 |
| Title : Carlactone-type strigolactones and their synthetic analogues as inducers of hyphal branching in arbuscular mycorrhizal fungi - Mori_2016_Phytochemistry_130_90 |
| Author(s) : Mori N , Nishiuma K , Sugiyama T , Hayashi H , Akiyama K |
| Ref : Phytochemistry , 130 :90 , 2016 |
| Abstract : Mori_2016_Phytochemistry_130_90 |
| ESTHER : Mori_2016_Phytochemistry_130_90 |
| PubMedSearch : Mori_2016_Phytochemistry_130_90 |
| PubMedID: 27264641 |
| Title : Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro - Abe_2014_Proc.Natl.Acad.Sci.U.S.A_111_18084 |
| Author(s) : Abe S , Sado A , Tanaka K , Kisugi T , Asami K , Ota S , Kim HI , Yoneyama K , Xie X , Ohnishi T , Seto Y , Yamaguchi S , Akiyama K , Nomura T |
| Ref : Proc Natl Acad Sci U S A , 111 :18084 , 2014 |
| Abstract : Abe_2014_Proc.Natl.Acad.Sci.U.S.A_111_18084 |
| ESTHER : Abe_2014_Proc.Natl.Acad.Sci.U.S.A_111_18084 |
| PubMedSearch : Abe_2014_Proc.Natl.Acad.Sci.U.S.A_111_18084 |
| PubMedID: 25425668 |
| Gene_locus related to this paper: arath-AtD14 |