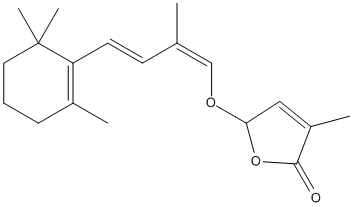
Carlactone
General
Type : Butenolide || Strigolactone receptors ligand || Non-canonical strigolactone
Chemical_Nomenclature : 4-methyl-2-[(1Z,3E)-2-methyl-4-(2,6,6-trimethylcyclohexen-1-yl)buta-1,3-dienoxy]-2H-furan-5-one
Canonical SMILES : CC1=C(C(CCC1)(C)C)C=CC(=COC2C=C(C(=O)O2)C)C
InChI : InChI=1S\/C19H26O3\/c1-13(12-21-17-11-15(3)18(20)22-17)8-9-16-14(2)7-6-10-19(16,4)5\/h8-9,11-12,17H,6-7,10H2,1-5H3\/b9-8+,13-12-
InChIKey : OTIYLZVFQIMLQH-FRGMPSNRSA-N
Other name(s) : 3-methyl-5-{[(1Z,3E)-2-methyl-4-(2,6,6-trimethylcyclohex-1-en-1-yl)buta-1,3-dien-1-yl]oxy}furan-2(5H)-one, SCHEMBL16116993, CHEBI:67190
MW : 302.4
Formula : C19H26O3
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : RsbQ-like
References (13)
| Title : CYP722C from Gossypium arboreum catalyzes the conversion of carlactonoic acid to 5-deoxystrigol - Wakabayashi_2020_Planta_251_97 |
| Author(s) : Wakabayashi T , Shida K , Kitano Y , Takikawa H , Mizutani M , Sugimoto Y |
| Ref : Planta , 251 :97 , 2020 |
| Abstract : Wakabayashi_2020_Planta_251_97 |
| ESTHER : Wakabayashi_2020_Planta_251_97 |
| PubMedSearch : Wakabayashi_2020_Planta_251_97 |
| PubMedID: 32306106 |
| Title : The tomato MAX1 homolog, SlMAX1, is involved in the biosynthesis of tomato strigolactones from carlactone - Zhang_2018_New.Phytol_219_297 |
| Author(s) : Zhang Y , Cheng X , Wang Y , Diez-Simon C , Flokova K , Bimbo A , Bouwmeester HJ , Ruyter-Spira C |
| Ref : New Phytol , 219 :297 , 2018 |
| Abstract : Zhang_2018_New.Phytol_219_297 |
| ESTHER : Zhang_2018_New.Phytol_219_297 |
| PubMedSearch : Zhang_2018_New.Phytol_219_297 |
| PubMedID: 29655242 |
| Title : Evidence for species-dependent biosynthetic pathways for converting carlactone to strigolactones in plants - Iseki_2018_J.Exp.Bot_69_2305 |
| Author(s) : Iseki M , Shida K , Kuwabara K , Wakabayashi T , Mizutani M , Takikawa H , Sugimoto Y |
| Ref : J Exp Bot , 69 :2305 , 2018 |
| Abstract : Iseki_2018_J.Exp.Bot_69_2305 |
| ESTHER : Iseki_2018_J.Exp.Bot_69_2305 |
| PubMedSearch : Iseki_2018_J.Exp.Bot_69_2305 |
| PubMedID: 29294064 |
| Title : Conversion of carlactone to carlactonoic acid is a conserved function of MAX1 homologs in strigolactone biosynthesis - Yoneyama_2018_New.Phytol_218_1522 |
| Author(s) : Yoneyama K , Mori N , Sato T , Yoda A , Xie X , Okamoto M , Iwanaga M , Ohnishi T , Nishiwaki H , Asami T , Yokota T , Akiyama K , Nomura T |
| Ref : New Phytol , 218 :1522 , 2018 |
| Abstract : Yoneyama_2018_New.Phytol_218_1522 |
| ESTHER : Yoneyama_2018_New.Phytol_218_1522 |
| PubMedSearch : Yoneyama_2018_New.Phytol_218_1522 |
| PubMedID: 29479714 |
| Title : Insights into the formation of carlactone from in-depth analysis of the CCD8-catalyzed reactions - Bruno_2017_FEBS.Lett_591_792 |
| Author(s) : Bruno M , Vermathen M , Alder A , Wust F , Schaub P , van der Steen R , Beyer P , Ghisla S , Al-Babili S |
| Ref : FEBS Letters , 591 :792 , 2017 |
| Abstract : Bruno_2017_FEBS.Lett_591_792 |
| ESTHER : Bruno_2017_FEBS.Lett_591_792 |
| PubMedSearch : Bruno_2017_FEBS.Lett_591_792 |
| PubMedID: 28186640 |
| Title : Carlactone-type strigolactones and their synthetic analogues as inducers of hyphal branching in arbuscular mycorrhizal fungi - Mori_2016_Phytochemistry_130_90 |
| Author(s) : Mori N , Nishiuma K , Sugiyama T , Hayashi H , Akiyama K |
| Ref : Phytochemistry , 130 :90 , 2016 |
| Abstract : Mori_2016_Phytochemistry_130_90 |
| ESTHER : Mori_2016_Phytochemistry_130_90 |
| PubMedSearch : Mori_2016_Phytochemistry_130_90 |
| PubMedID: 27264641 |
| Title : Nitro-Phenlactone, a Carlactone Analog with Pleiotropic Strigolactone Activities - |
| Author(s) : Jia KP , Kountche BA , Jamil M , Guo X , Ntui VO , Rufenacht A , Rochange S , Al-Babili S |
| Ref : Mol Plant , 9 :1341 , 2016 |
| PubMedID: 27288318 |
| Title : Heliolactone, a non-sesquiterpene lactone germination stimulant for root parasitic weeds from sunflower - Ueno_2014_Phytochemistry_108_122 |
| Author(s) : Ueno K , Furumoto T , Umeda S , Mizutani M , Takikawa H , Batchvarova R , Sugimoto Y |
| Ref : Phytochemistry , 108 :122 , 2014 |
| Abstract : Ueno_2014_Phytochemistry_108_122 |
| ESTHER : Ueno_2014_Phytochemistry_108_122 |
| PubMedSearch : Ueno_2014_Phytochemistry_108_122 |
| PubMedID: 25446236 |
| Title : Carlactone is an endogenous biosynthetic precursor for strigolactones - Seto_2014_Proc.Natl.Acad.Sci.U.S.A_111_1640 |
| Author(s) : Seto Y , Sado A , Asami K , Hanada A , Umehara M , Akiyama K , Yamaguchi S |
| Ref : Proc Natl Acad Sci U S A , 111 :1640 , 2014 |
| Abstract : Seto_2014_Proc.Natl.Acad.Sci.U.S.A_111_1640 |
| ESTHER : Seto_2014_Proc.Natl.Acad.Sci.U.S.A_111_1640 |
| PubMedSearch : Seto_2014_Proc.Natl.Acad.Sci.U.S.A_111_1640 |
| PubMedID: 24434551 |
| Title : Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis - Zhang_2014_Nat.Chem.Biol_10_1028 |
| Author(s) : Zhang Y , van Dijk AD , Scaffidi A , Flematti GR , Hofmann M , Charnikhova T , Verstappen F , Hepworth J , van der Krol S , Leyser O , Smith SM , Zwanenburg B , Al-Babili S , Ruyter-Spira C , Bouwmeester HJ |
| Ref : Nat Chemical Biology , 10 :1028 , 2014 |
| Abstract : Zhang_2014_Nat.Chem.Biol_10_1028 |
| ESTHER : Zhang_2014_Nat.Chem.Biol_10_1028 |
| PubMedSearch : Zhang_2014_Nat.Chem.Biol_10_1028 |
| PubMedID: 25344813 |
| Title : Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro - Abe_2014_Proc.Natl.Acad.Sci.U.S.A_111_18084 |
| Author(s) : Abe S , Sado A , Tanaka K , Kisugi T , Asami K , Ota S , Kim HI , Yoneyama K , Xie X , Ohnishi T , Seto Y , Yamaguchi S , Akiyama K , Nomura T |
| Ref : Proc Natl Acad Sci U S A , 111 :18084 , 2014 |
| Abstract : Abe_2014_Proc.Natl.Acad.Sci.U.S.A_111_18084 |
| ESTHER : Abe_2014_Proc.Natl.Acad.Sci.U.S.A_111_18084 |
| PubMedSearch : Abe_2014_Proc.Natl.Acad.Sci.U.S.A_111_18084 |
| PubMedID: 25425668 |
| Gene_locus related to this paper: arath-AtD14 |
| Title : Carlactone-independent seedling morphogenesis in Arabidopsis - Scaffidi_2013_Plant.J_76_1 |
| Author(s) : Scaffidi A , Waters MT , Ghisalberti EL , Dixon KW , Flematti GR , Smith SM |
| Ref : Plant J , 76 :1 , 2013 |
| Abstract : Scaffidi_2013_Plant.J_76_1 |
| ESTHER : Scaffidi_2013_Plant.J_76_1 |
| PubMedSearch : Scaffidi_2013_Plant.J_76_1 |
| PubMedID: 23773129 |
| Title : The path from beta-carotene to carlactone, a strigolactone-like plant hormone - Alder_2012_Science_335_1348 |
| Author(s) : Alder A , Jamil M , Marzorati M , Bruno M , Vermathen M , Bigler P , Ghisla S , Bouwmeester H , Beyer P , Al-Babili S |
| Ref : Science , 335 :1348 , 2012 |
| Abstract : Alder_2012_Science_335_1348 |
| ESTHER : Alder_2012_Science_335_1348 |
| PubMedSearch : Alder_2012_Science_335_1348 |
| PubMedID: 22422982 |