C6-HSL
General
Type : Lactone || Homoserine
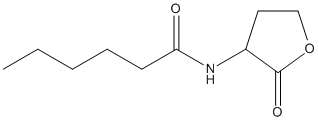
Chemical_Nomenclature : N-[(3S)-2-oxooxolan-3-yl]hexanamide
Canonical SMILES : CCCCCC(=O)NC1CCOC1=O
InChI : InChI=1S\/C10H17NO3\/c1-2-3-4-5-9(12)11-8-6-7-14-10(8)13\/h8H,2-7H2,1H3,(H,11,12)\/t8-\/m0\/s1
InChIKey : ZJFKKPDLNLCPNP-QMMMGPOBSA-N
Other name(s) : SCHEMBL12341577, ZINC6655698, N-hexanoyl homoserine lactone, N-hexanoyl-L-Homoserine lactone, N-[(3S)-Tetrahydro-2-oxo-3-furanyl]-hexanamide, N-[(3s)-2-Oxotetrahydrofuran-3-Yl]hexanamide, HL6, HHSL
Target
Families : AHL-acylase, 6_AlphaBeta_hydrolase
References (2)
| Title : Molecular and functional basis of a novel Amazonian Dark Earth Esterase 1 (Ade1) with hysteresis behavior and quorum-quenching activity - Vinces_2021_Biorxiv__ |
| Author(s) : Vinces TC , de Souza AS , Carvalho CF , Teixeira RD , Bismara BAP , Vicente EJ , Pereira JO , de Souza RF , Yonamine M , Marana SR , Farah CS , Guzzo CR |
| Ref : Biorxiv , : , 2021 |
| Abstract : Vinces_2021_Biorxiv__ |
| ESTHER : Vinces_2021_Biorxiv__ |
| PubMedSearch : Vinces_2021_Biorxiv__ |
| PubMedID: |
| Gene_locus related to this paper: 9bact-6EB3 |
| Title : High-resolution structures of AidH complexes provide insights into a novel catalytic mechanism for N-acyl homoserine lactonase - Gao_2013_Acta.Crystallogr.D.Biol.Crystallogr_69_82 |
| Author(s) : Gao A , Mei GY , Liu S , Wang P , Tang Q , Liu YP , Wen H , An XM , Zhang LQ , Yan XX , Liang DC |
| Ref : Acta Crystallographica D Biol Crystallogr , 69 :82 , 2013 |
| Abstract : Gao_2013_Acta.Crystallogr.D.Biol.Crystallogr_69_82 |
| ESTHER : Gao_2013_Acta.Crystallogr.D.Biol.Crystallogr_69_82 |
| PubMedSearch : Gao_2013_Acta.Crystallogr.D.Biol.Crystallogr_69_82 |
| PubMedID: 23275166 |
| Gene_locus related to this paper: 9rhiz-d2j2t6 |