C6-CoA
General
Type : CoA || Sulfur compound
Chemical_Nomenclature :
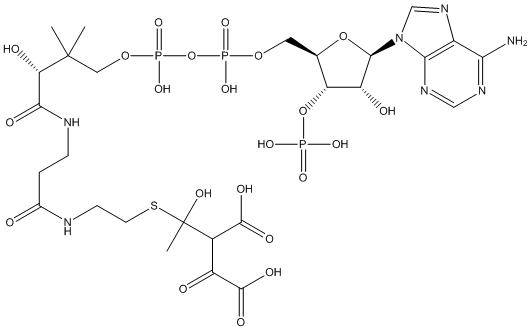
Canonical SMILES : C12=C(C(=N[C]=N1)N)N=[C][N]2[C]3[C]([C]([C](O3)CO[P]([O])(=O)O[P](=O)([O])OCC([C](C([N]CCC([N]CCSC([C])([O])[C](C(=O)[O])C(=O)C(=O)[O])=O)=O)[O])([C])[C])O[P]([O])([O])=O)[O]
InChI : InChI=1S\/C27H20N7O22P3S\/c1-26(2,19(38)22(39)30-5-4-13(35)29-6-7-60-27(3,44)14(24(40)41)16(36)25(42)43)9-53-59(50,51)56-58(48,49)52-8-12-18(55-57(45,46)47)17(37)23(54-12)34-11-33-15-20(28)31-10-32-21(15)34\/h1-9H2,(H2,28,31,32)
InChIKey : VAGBQFIAKQENGK-UHFFFAOYSA-N
Other name(s) : 2-Acetyl-3-oxobutanedioic-CoA
MW : 919.47
Formula : C27H20N7O22P3S
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : AlphaBeta_hydrolase
References (2)
| Title : Structural Insights into an Oxalate-producing Serine Hydrolase with an Unusual Oxyanion Hole and Additional Lyase Activity - Oh_2016_J.Biol.Chem_291_15185 |
| Author(s) : Oh J , Hwang I , Rhee S |
| Ref : Journal of Biological Chemistry , 291 :15185 , 2016 |
| Abstract : Oh_2016_J.Biol.Chem_291_15185 |
| ESTHER : Oh_2016_J.Biol.Chem_291_15185 |
| PubMedSearch : Oh_2016_J.Biol.Chem_291_15185 |
| PubMedID: 27226606 |
| Gene_locus related to this paper: burta-A0A096YSN3 |
| Title : The oxalic acid biosynthetic activity of Burkholderia mallei is encoded by a single locus - Nakata_2011_Microbiol.Res_166_531 |
| Author(s) : Nakata PA |
| Ref : Microbiol Res , 166 :531 , 2011 |
| Abstract : Nakata_2011_Microbiol.Res_166_531 |
| ESTHER : Nakata_2011_Microbiol.Res_166_531 |
| PubMedSearch : Nakata_2011_Microbiol.Res_166_531 |
| PubMedID: 21242070 |
| Gene_locus related to this paper: burta-A0A096YSN3 |