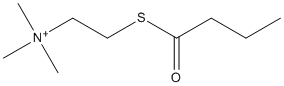
Butyrylthiocholine
General
Type : Butyrate || Trimethylammonium || Choline ester || Chromogen
Chemical_Nomenclature : 2-butanoylsulfanylethyl(trimethyl)azanium
Canonical SMILES : CCCC(=O)SCC[N+](C)(C)C
InChI : InChI=1S\/C9H20NOS\/c1-5-6-9(11)12-8-7-10(2,3)4\/h5-8H2,1-4H3\/q+1
InChIKey : AWBGQVBMGBZGLS-UHFFFAOYSA-N
Other name(s) : CHEMBL139908, 2-(butanoylsulfanyl)-N,N,N-trimethylethanaminium, 2-(BUTYRYLSULFANYL)-N,N,N-TRIMETHYLETHANAMINIUM, CHEMBL148530, BTC
Target
Families : BCHE
References (8)
| Title : Substrate and product trafficking through the active center gorge of acetylcholinesterase analyzed by crystallography and equilibrium binding - Bourne_2006_J.Biol.Chem_281_29256 |
| Author(s) : Bourne Y , Radic Z , Sulzenbacher G , Kim E , Taylor P , Marchot P |
| Ref : Journal of Biological Chemistry , 281 :29256 , 2006 |
| Abstract : Bourne_2006_J.Biol.Chem_281_29256 |
| ESTHER : Bourne_2006_J.Biol.Chem_281_29256 |
| PubMedSearch : Bourne_2006_J.Biol.Chem_281_29256 |
| PubMedID: 16837465 |
| Gene_locus related to this paper: mouse-ACHE |
| Title : Effects of mutations of active site residues and amino acids interacting with the Omega loop on substrate activation of butyrylcholinesterase - Masson_2001_Biochim.Biophys.Acta_1544_166 |
| Author(s) : Masson P , Xie W , Froment MT , Lockridge O |
| Ref : Biochimica & Biophysica Acta , 1544 :166 , 2001 |
| Abstract : Masson_2001_Biochim.Biophys.Acta_1544_166 |
| ESTHER : Masson_2001_Biochim.Biophys.Acta_1544_166 |
| PubMedSearch : Masson_2001_Biochim.Biophys.Acta_1544_166 |
| PubMedID: 11341926 |
| Gene_locus related to this paper: human-BCHE |
| Title : Fasciculin 2 binds to the peripheral site on acetylcholinesterase and inhibits substrate hydrolysis by slowing a step involving proton transfer during enzyme acylation - Eastman_1995_J.Biol.Chem_270_19694 |
| Author(s) : Eastman J , Wilson EJ , Cervenansky C , Rosenberry TL |
| Ref : Journal of Biological Chemistry , 270 :19694 , 1995 |
| Abstract : Eastman_1995_J.Biol.Chem_270_19694 |
| ESTHER : Eastman_1995_J.Biol.Chem_270_19694 |
| PubMedSearch : Eastman_1995_J.Biol.Chem_270_19694 |
| PubMedID: 7649979 |
| Title : Site-directed mutagenesis of active site residues reveals plasticity of human butyrylcholinesterase in substrate and inhibitor interactions - Gnatt_1994_J.Neurochem_62_749 |
| Author(s) : Gnatt A , Loewenstein Y , Yaron A , Schwarz M , Soreq H |
| Ref : Journal of Neurochemistry , 62 :749 , 1994 |
| Abstract : Gnatt_1994_J.Neurochem_62_749 |
| ESTHER : Gnatt_1994_J.Neurochem_62_749 |
| PubMedSearch : Gnatt_1994_J.Neurochem_62_749 |
| PubMedID: 8294937 |
| Title : [Comparative characterization of the catalytic properties of blood serum cholinesterase in pigeon and hen]. [Russian] - Zhukovskii_1994_Ukrainskii.Biokhimicheskii.Zhurnal_66_23 |
| Author(s) : Zhukovskii Iu G , Kuznestova LP , Sochilina EE , Fartseiger AG , Fartseiger NL , Azliarova MA |
| Ref : Ukrainskii Biokhimicheskii Zhurnal , 66 :23 , 1994 |
| Abstract : Zhukovskii_1994_Ukrainskii.Biokhimicheskii.Zhurnal_66_23 |
| ESTHER : Zhukovskii_1994_Ukrainskii.Biokhimicheskii.Zhurnal_66_23 |
| PubMedSearch : Zhukovskii_1994_Ukrainskii.Biokhimicheskii.Zhurnal_66_23 |
| PubMedID: 7747340 |
| Title : Application of stepwise discriminant analysis in the phenotyping of plasma cholinesterase variants - Turner_1985_Ann.Clin.Biochem_22_175 |
| Author(s) : Turner JM , Hall RA , Whittaker M , Holder RL , Kricka LJ |
| Ref : Annals of Clinical Biochemistry , 22 :175 , 1985 |
| Abstract : Turner_1985_Ann.Clin.Biochem_22_175 |
| ESTHER : Turner_1985_Ann.Clin.Biochem_22_175 |
| PubMedSearch : Turner_1985_Ann.Clin.Biochem_22_175 |
| PubMedID: 4004108 |
| Title : Polymorphism of pseudocholinesterase in Torpedo marmorata tissues: comparative study of the catalytic and molecular properties of this enzyme with acetylcholinesterase - Toutant_1985_J.Neurochem_44_580 |
| Author(s) : Toutant JP , Massoulie J , Bon S |
| Ref : Journal of Neurochemistry , 44 :580 , 1985 |
| Abstract : Toutant_1985_J.Neurochem_44_580 |
| ESTHER : Toutant_1985_J.Neurochem_44_580 |
| PubMedSearch : Toutant_1985_J.Neurochem_44_580 |
| PubMedID: 2578181 |
| Title : Comparison of a commercially available assay system with two reference methods for the determination of plasma cholinesterase variants - Whittaker_1983_Clin.Chem_29_1746 |
| Author(s) : Whittaker M , Britten JJ , Dawson PJ |
| Ref : Clinical Chemistry , 29 :1746 , 1983 |
| Abstract : Whittaker_1983_Clin.Chem_29_1746 |
| ESTHER : Whittaker_1983_Clin.Chem_29_1746 |
| PubMedSearch : Whittaker_1983_Clin.Chem_29_1746 |
| PubMedID: 6616819 |