Buibuilactone
General
Type : Odorant || Pheromone || Lactone
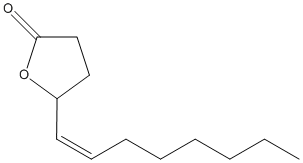
Chemical_Nomenclature : (5R)-5-[(Z)-oct-1-enyl]oxolan-2-one
Canonical SMILES : CCCCCCC=CC1CCC(=O)O1
InChI : InChI=1S\/C12H20O2\/c1-2-3-4-5-6-7-8-11-9-10-12(13)14-11\/h7-8,11H,2-6,9-10H2,1H3\/b8-7-\/t11-\/m0\/s1
InChIKey : ADZMLQKJPQFTIS-TVRMLOFPSA-N
Other name(s) : Dodec-5Z-en-4R-olide, 5R-(Oct-1Z-enyl)-oxacyclopentan-2-one, 5R-(oct-1Z-enyl)dihydrofuran-2(3H)-one, (R,Z)-5-dodecen-4-olide, SCHEMBL5148270
MW : 196.29
Formula : C12H20O2
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : No family
References (4)
| Title : The chafer pheromone buibuilactone and ant pyrazines are also produced by marine bacteria - Dickschat_2005_J.Chem.Ecol_31_925 |
| Author(s) : Dickschat JS , Wagner-Dobler I , Schulz S |
| Ref : J Chem Ecol , 31 :925 , 2005 |
| Abstract : Dickschat_2005_J.Chem.Ecol_31_925 |
| ESTHER : Dickschat_2005_J.Chem.Ecol_31_925 |
| PubMedSearch : Dickschat_2005_J.Chem.Ecol_31_925 |
| PubMedID: 16124260 |
| Title : A sex attractant for the scarab beetle Anomala solida Er - Toth_2003_J.Chem.Ecol_29_1643 |
| Author(s) : Toth M , Subchev M , Sredkov I , Szarukan I , Leal W |
| Ref : J Chem Ecol , 29 :1643 , 2003 |
| Abstract : Toth_2003_J.Chem.Ecol_29_1643 |
| ESTHER : Toth_2003_J.Chem.Ecol_29_1643 |
| PubMedSearch : Toth_2003_J.Chem.Ecol_29_1643 |
| PubMedID: 12921442 |
| Title : Biosynthesis of scarab beetle pheromones - Leal_1999_Eur.J.Biochem_259_175 |
| Author(s) : Leal WS , Zarbin PH , Wojtasek H , Ferreira JT |
| Ref : European Journal of Biochemistry , 259 :175 , 1999 |
| Abstract : Leal_1999_Eur.J.Biochem_259_175 |
| ESTHER : Leal_1999_Eur.J.Biochem_259_175 |
| PubMedSearch : Leal_1999_Eur.J.Biochem_259_175 |
| PubMedID: 9914490 |
| Title : The scarab beetleAnomala albopilosa sakishimana utilizes the same sex pheromone blend as a closely related and geographically isolated species,Anomala cuprea - Leal_1994_J.Chem.Ecol_20_1667 |
| Author(s) : Leal WS , Kawamura F , Ono M |
| Ref : J Chem Ecol , 20 :1667 , 1994 |
| Abstract : Leal_1994_J.Chem.Ecol_20_1667 |
| ESTHER : Leal_1994_J.Chem.Ecol_20_1667 |
| PubMedSearch : Leal_1994_J.Chem.Ecol_20_1667 |
| PubMedID: 24242659 |