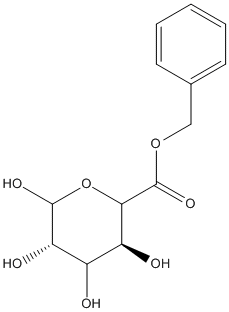
Benzyl-D-glucuronate
General
Type : Glucuronoyl || Carbohydrate || Glycoside
Chemical_Nomenclature : benzyl (3S,5S)-3,4,5,6-tetrahydroxyoxane-2-carboxylate
Canonical SMILES : C1=CC=C(C=C1)COC(=O)C2C(C(C(C(O2)O)O)O)O
InChI : InChI=1S\/C13H16O7\/c14-8-9(15)11(20-12(17)10(8)16)13(18)19-6-7-4-2-1-3-5-7\/h1-5,8-12,14-17H,6H2\/t8?,9-,10-,11?,12?\/m0\/s1
InChIKey : MYEUFSLWFIOAGY-SJZSRGDHSA-N
Other name(s) : BnGlcA, BnzGlcA, Glucuronic acid benzyl ester, D-Glucuronic acid phenylmethyl ester, Benzyl D-Glucuronate, CTK8F1098, RT-011473
Target
Families : Glucuronoyl_esterase
References (6)
| Title : Structural and functional investigation of a fungal member of carbohydrate esterase family 15 with potential specificity for rare xylans - Mazurkewich_2023_Acta.Crystallogr.D.Struct.Biol__ |
| Author(s) : Mazurkewich S , Scholzen KC , Brusch RH , Poulsen JCN , Theibich Y , Huttner S , Olsson L , Larsbrink J , Lo Leggio L |
| Ref : Acta Crystallographica D Struct Biol , : , 2023 |
| Abstract : Mazurkewich_2023_Acta.Crystallogr.D.Struct.Biol__ |
| ESTHER : Mazurkewich_2023_Acta.Crystallogr.D.Struct.Biol__ |
| PubMedSearch : Mazurkewich_2023_Acta.Crystallogr.D.Struct.Biol__ |
| PubMedID: 37227091 |
| Gene_locus related to this paper: 9pleo-LfCE15C |
| Title : Expression and characterization of two glucuronoyl esterases from Thielavia terrestris and their application in enzymatic hydrolysis of corn bran - Tang_2019_Appl.Microbiol.Biotechnol_103_3037 |
| Author(s) : Tang J , Long L , Cao Y , Ding S |
| Ref : Applied Microbiology & Biotechnology , 103 :3037 , 2019 |
| Abstract : Tang_2019_Appl.Microbiol.Biotechnol_103_3037 |
| ESTHER : Tang_2019_Appl.Microbiol.Biotechnol_103_3037 |
| PubMedSearch : Tang_2019_Appl.Microbiol.Biotechnol_103_3037 |
| PubMedID: 30762074 |
| Gene_locus related to this paper: thite-g2r8b5 , thite-g2rcm8 |
| Title : Fungal glucuronoyl esterases: Genome mining based enzyme discovery and biochemical characterization - Dilokpimol_2018_N.Biotechnol_40_282 |
| Author(s) : Dilokpimol A , Makela MR , Cerullo G , Zhou M , Varriale S , Gidijala L , Bras JLA , Jutten P , Piechot A , Verhaert R , Faraco V , Hilden KS , de Vries RP |
| Ref : N Biotechnol , 40 :282 , 2018 |
| Abstract : Dilokpimol_2018_N.Biotechnol_40_282 |
| ESTHER : Dilokpimol_2018_N.Biotechnol_40_282 |
| PubMedSearch : Dilokpimol_2018_N.Biotechnol_40_282 |
| PubMedID: 29051046 |
| Title : Characterisation of three fungal glucuronoyl esterases on glucuronic acid ester model compounds - Huttner_2017_Appl.Microbiol.Biotechnol_101_5301 |
| Author(s) : Huttner S , Klaubauf S , de Vries RP , Olsson L |
| Ref : Applied Microbiology & Biotechnology , 101 :5301 , 2017 |
| Abstract : Huttner_2017_Appl.Microbiol.Biotechnol_101_5301 |
| ESTHER : Huttner_2017_Appl.Microbiol.Biotechnol_101_5301 |
| PubMedSearch : Huttner_2017_Appl.Microbiol.Biotechnol_101_5301 |
| PubMedID: 28429057 |
| Gene_locus related to this paper: wolco-gce1 , acram-a0a1d8ejg8 , phacr-gce1 , phacr-gce2 |
| Title : beta-Glucuronidase-coupled assays of glucuronoyl esterases - Franova_2016_Anal.Biochem_510_114 |
| Author(s) : Franova L , Puchart V , Biely P |
| Ref : Analytical Biochemistry , 510 :114 , 2016 |
| Abstract : Franova_2016_Anal.Biochem_510_114 |
| ESTHER : Franova_2016_Anal.Biochem_510_114 |
| PubMedSearch : Franova_2016_Anal.Biochem_510_114 |
| PubMedID: 27452816 |
| Title : Glucuronoyl Esterase Screening and Characterization Assays Utilizing Commercially Available Benzyl Glucuronic Acid Ester - Sunner_2015_Molecules_20_17807 |
| Author(s) : Sunner H , Charavgi MD , Olsson L , Topakas E , Christakopoulos P |
| Ref : Molecules , 20 :17807 , 2015 |
| Abstract : Sunner_2015_Molecules_20_17807 |
| ESTHER : Sunner_2015_Molecules_20_17807 |
| PubMedSearch : Sunner_2015_Molecules_20_17807 |
| PubMedID: 26404219 |