Acetylcholine
General
Type : Acetate || Trimethylammonium || Natural || Choline ester
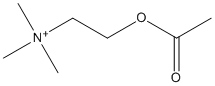
Chemical_Nomenclature : 2-(Acetyloxy)-N,N,N,-trimethylethanaminium
Canonical SMILES : CC(=O)OCC[N+](C)(C)C
InChI : InChI=1S\/C7H16NO2\/c1-7(9)10-6-5-8(2,3)4\/h5-6H2,1-4H3\/q+1
InChIKey : OIPILFWXSMYKGL-UHFFFAOYSA-N
Other name(s) : Choline acetate, O-Acetylcholine, Acetyl choline ion, Choline acetate (ester), Acetylcholinum
Target
Families : ACHE, BCHE, Cholinesterase, Cholinesterase-like
References (6)
| Title : Substrate and product trafficking through the active center gorge of acetylcholinesterase analyzed by crystallography and equilibrium binding - Bourne_2006_J.Biol.Chem_281_29256 |
| Author(s) : Bourne Y , Radic Z , Sulzenbacher G , Kim E , Taylor P , Marchot P |
| Ref : Journal of Biological Chemistry , 281 :29256 , 2006 |
| Abstract : Bourne_2006_J.Biol.Chem_281_29256 |
| ESTHER : Bourne_2006_J.Biol.Chem_281_29256 |
| PubMedSearch : Bourne_2006_J.Biol.Chem_281_29256 |
| PubMedID: 16837465 |
| Gene_locus related to this paper: mouse-ACHE |
| Title : Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A - Raves_1997_Nat.Struct.Biol_4_57 |
| Author(s) : Raves ML , Harel M , Pang YP , Silman I , Kozikowski AP , Sussman JL |
| Ref : Nat Struct Biol , 4 :57 , 1997 |
| Abstract : Raves_1997_Nat.Struct.Biol_4_57 |
| ESTHER : Raves_1997_Nat.Struct.Biol_4_57 |
| PubMedSearch : Raves_1997_Nat.Struct.Biol_4_57 |
| PubMedID: 8989325 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein - Sussman_1991_Science_253_872 |
| Author(s) : Sussman JL , Harel M , Frolow F , Oefner C , Goldman A , Toker L , Silman I |
| Ref : Science , 253 :872 , 1991 |
| Abstract : Sussman_1991_Science_253_872 |
| ESTHER : Sussman_1991_Science_253_872 |
| PubMedSearch : Sussman_1991_Science_253_872 |
| PubMedID: 1678899 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |