Acetyl-CoA
General
Type : CoA || Aminopurin
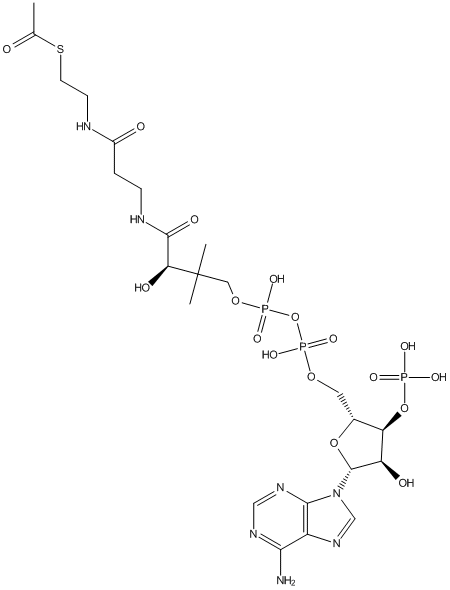
Chemical_Nomenclature : S-[2-[3-[[(2R)-4-[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,3-dimethylbutanoyl]amino]propanoylamino]ethyl] ethanethioate
Canonical SMILES : CC(=O)SCCNC(=O)CCNC(=O)C(C(C)(C)COP(=O)(O)OP(=O)(O)OCC1C(C(C(O1)N2C=NC3=C2N=CN=C3N)O)OP(=O)(O)O)O
InChI : InChI=1S\/C23H38N7O17P3S\/c1-12(31)51-7-6-25-14(32)4-5-26-21(35)18(34)23(2,3)9-44-50(41,42)47-49(39,40)43-8-13-17(46-48(36,37)38)16(33)22(45-13)30-11-29-15-19(24)27-10-28-20(15)30\/h10-11,13,16-18,22,33-34H,4-9H2,1-3H3,(H,25,32)(H,26,35)(H,39,40)(H,41,42)(H2,24,27,28)(H2,36,37,38)\/t13-,16-,17-,18+,22-\/m1\/s1
InChIKey : ZSLZBFCDCINBPY-ZSJPKINUSA-N
Other name(s) : ACETYL COENZYME A, S-Acetyl coenzyme A, AcCoA, Acetyl-S-CoA, S-acetyl-CoA
MW : 809.57
Formula : C23H38N7O17P3S
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Homoserine_transacetylase, AlphaBeta_hydrolase
References (3)
| Title : Structural and mechanistic basis of capsule O-acetylation in Neisseria meningitidis serogroup A - Fiebig_2020_Nat.Commun_11_4723 |
| Author(s) : Fiebig T , Cramer JT , Bethe A , Baruch P , Curth U , Fuhring JI , Buettner FFR , Vogel U , Schubert M , Fedorov R , Muhlenhoff M |
| Ref : Nat Commun , 11 :4723 , 2020 |
| Abstract : Fiebig_2020_Nat.Commun_11_4723 |
| ESTHER : Fiebig_2020_Nat.Commun_11_4723 |
| PubMedSearch : Fiebig_2020_Nat.Commun_11_4723 |
| PubMedID: 32948778 |
| Gene_locus related to this paper: neime-r0tza2 |
| Title : The last step in cephalosporin C formation revealed: crystal structures of deacetylcephalosporin C acetyltransferase from Acremonium chrysogenum in complexes with reaction intermediates - Lejon_2008_J.Mol.Biol_377_935 |
| Author(s) : Lejon S , Ellis J , Valegard K |
| Ref : Journal of Molecular Biology , 377 :935 , 2008 |
| Abstract : Lejon_2008_J.Mol.Biol_377_935 |
| ESTHER : Lejon_2008_J.Mol.Biol_377_935 |
| PubMedSearch : Lejon_2008_J.Mol.Biol_377_935 |
| PubMedID: 18279889 |
| Gene_locus related to this paper: cepac-cefg |
| Title : Catalytic mechanism of fungal homoserine transacetylase - Nazi_2005_Biochemistry_44_13560 |
| Author(s) : Nazi I , Wright GD |
| Ref : Biochemistry , 44 :13560 , 2005 |
| Abstract : Nazi_2005_Biochemistry_44_13560 |
| ESTHER : Nazi_2005_Biochemistry_44_13560 |
| PubMedSearch : Nazi_2005_Biochemistry_44_13560 |
| PubMedID: 16216079 |