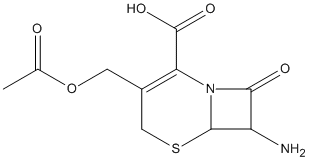
7-Aminocephalosporanic-acid
General
Type : Natural || Antibiotic || Azabicyclo
Chemical_Nomenclature : (6R,7R)-3-(acetyloxymethyl)-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Canonical SMILES : CC(=O)OCC1=C(N2C(C(C2=O)N)SC1)C(=O)O
InChI : InChI=1S\/C10H12N2O5S\/c1-4(13)17-2-5-3-18-9-6(11)8(14)12(9)7(5)10(15)16\/h6,9H,2-3,11H2,1H3,(H,15,16)\/t6-,9-\/m1\/s1
InChIKey : HSHGZXNAXBPPDL-HZGVNTEJSA-N
Other name(s) : 7-Aminocephalosporanic acid, 957-68-6, 7-ACA, 7-Aminocephalosporinic acid, CHEBI:2255, (7R)-7-Aminocephalosporanate
MW : 272.27
Formula : C10H12N2O5S
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Acetyl-esterase_deacetylase, BD-FAE
References (11)
| Title : Characterization of EstZY: A new acetylesterase with 7-aminocephalosporanic acid deacetylase activity from Alicyclobacillus tengchongensis - Ding_2020_Int.J.Biol.Macromol_148_333 |
| Author(s) : Ding J , Zhou Y , Zhu H , Deng M , Gao Y , Yang Y , Huang Z |
| Ref : Int J Biol Macromol , 148 :333 , 2020 |
| Abstract : Ding_2020_Int.J.Biol.Macromol_148_333 |
| ESTHER : Ding_2020_Int.J.Biol.Macromol_148_333 |
| PubMedSearch : Ding_2020_Int.J.Biol.Macromol_148_333 |
| PubMedID: 31954783 |
| Gene_locus related to this paper: 9bacl-a0a6g6c491 |
| Title : Identification and characterization of an acetyl esterase from Paenibacillus sp. XW-6-66 and its novel function in 7-aminocephalosporanic acid deacetylation - Ding_2019_Biotechnol.Lett_41_1059 |
| Author(s) : Ding J , Zhou Y , Zhu H , Deng M , Long L , Yang Y , Wu Q , Huang Z |
| Ref : Biotechnol Lett , 41 :1059 , 2019 |
| Abstract : Ding_2019_Biotechnol.Lett_41_1059 |
| ESTHER : Ding_2019_Biotechnol.Lett_41_1059 |
| PubMedSearch : Ding_2019_Biotechnol.Lett_41_1059 |
| PubMedID: 31302814 |
| Gene_locus related to this paper: 9bacl-7ACAaes |
| Title : Crystal structure and functional characterization of a cold-active acetyl xylan esterase (PbAcE) from psychrophilic soil microbe Paenibacillus sp - Park_2018_PLoS.One_13_e0206260 |
| Author(s) : Park SH , Yoo W , Lee CW , Jeong CS , Shin SC , Kim HW , Park H , Kim KK , Kim TD , Lee JH |
| Ref : PLoS ONE , 13 :e0206260 , 2018 |
| Abstract : Park_2018_PLoS.One_13_e0206260 |
| ESTHER : Park_2018_PLoS.One_13_e0206260 |
| PubMedSearch : Park_2018_PLoS.One_13_e0206260 |
| PubMedID: 30379876 |
| Gene_locus related to this paper: 9mico-6AGQ |
| Title : Role of an N-terminal extension in stability and catalytic activity of a hyperthermostable alpha\/beta hydrolase fold esterase - Singh_2017_Protein.Eng.Des.Sel_30_559 |
| Author(s) : Singh MK , Shivakumaraswamy S , Gummadi SN , Manoj N |
| Ref : Protein Engineering Des Sel , 30 :559 , 2017 |
| Abstract : Singh_2017_Protein.Eng.Des.Sel_30_559 |
| ESTHER : Singh_2017_Protein.Eng.Des.Sel_30_559 |
| PubMedSearch : Singh_2017_Protein.Eng.Des.Sel_30_559 |
| PubMedID: 28967962 |
| Gene_locus related to this paper: thema-TM0077 |
| Title : An extended loop in CE7 carbohydrate esterase family is dispensable for oligomerization but required for activity and thermostability - Singh_2016_J.Struct.Biol_194_434 |
| Author(s) : Singh MK , Manoj N |
| Ref : J Struct Biol , 194 :434 , 2016 |
| Abstract : Singh_2016_J.Struct.Biol_194_434 |
| ESTHER : Singh_2016_J.Struct.Biol_194_434 |
| PubMedSearch : Singh_2016_J.Struct.Biol_194_434 |
| PubMedID: 27085421 |
| Gene_locus related to this paper: thema-TM0077 |
| Title : A novel cephalosporin deacetylating acetyl xylan esterase from Bacillus subtilis with high activity toward cephalosporin C and 7-aminocephalosporanic acid - Tian_2014_Appl.Microbiol.Biotechnol_98_2081 |
| Author(s) : Tian Q , Song P , Jiang L , Li S , Huang H |
| Ref : Applied Microbiology & Biotechnology , 98 :2081 , 2014 |
| Abstract : Tian_2014_Appl.Microbiol.Biotechnol_98_2081 |
| ESTHER : Tian_2014_Appl.Microbiol.Biotechnol_98_2081 |
| PubMedSearch : Tian_2014_Appl.Microbiol.Biotechnol_98_2081 |
| PubMedID: 23828600 |
| Title : The structural basis for the narrow substrate specificity of an acetyl esterase from Thermotoga maritima - Hedge_2012_Biochim.Biophys.Acta_1824_1024 |
| Author(s) : Hedge MK , Gehring AM , Adkins CT , Weston LA , Lavis LD , Johnson RJ |
| Ref : Biochimica & Biophysica Acta , 1824 :1024 , 2012 |
| Abstract : Hedge_2012_Biochim.Biophys.Acta_1824_1024 |
| ESTHER : Hedge_2012_Biochim.Biophys.Acta_1824_1024 |
| PubMedSearch : Hedge_2012_Biochim.Biophys.Acta_1824_1024 |
| PubMedID: 22659119 |
| Gene_locus related to this paper: thema-TM0077 |
| Title : A colorimetric assay for the determination of acetyl xylan esterase or cephalosporin C acetyl esterase activities using 7-amino cephalosporanic acid, cephalosporin C, or acetylated xylan as substrate - Martinez-Martinez_2007_Anal.Biochem_369_210 |
| Author(s) : Martinez-Martinez I , Montoro-Garcia S , Lozada-Ramirez JD , Sanchez-Ferrer A , Garcia-Carmona F |
| Ref : Analytical Biochemistry , 369 :210 , 2007 |
| Abstract : Martinez-Martinez_2007_Anal.Biochem_369_210 |
| ESTHER : Martinez-Martinez_2007_Anal.Biochem_369_210 |
| PubMedSearch : Martinez-Martinez_2007_Anal.Biochem_369_210 |
| PubMedID: 17651681 |
| Title : Batch production of deacetyl 7-aminocephalosporanic acid by immobilized cephalosporin-C deacetylase - Takimoto_2004_Appl.Microbiol.Biotechnol_65_263 |
| Author(s) : Takimoto A , Takakura T , Tani H , Yagi S , Mitsushima K |
| Ref : Applied Microbiology & Biotechnology , 65 :263 , 2004 |
| Abstract : Takimoto_2004_Appl.Microbiol.Biotechnol_65_263 |
| ESTHER : Takimoto_2004_Appl.Microbiol.Biotechnol_65_263 |
| PubMedSearch : Takimoto_2004_Appl.Microbiol.Biotechnol_65_263 |
| PubMedID: 15069587 |
| Gene_locus related to this paper: bacsu-CAH |
| Title : Isolation, analysis, and expression of two genes from Thermoanaerobacterium sp. strain JW\/SL YS485: a beta-xylosidase and a novel acetyl xylan esterase with cephalosporin C deacetylase activity - Lorenz_1997_J.Bacteriol_179_5436 |
| Author(s) : Lorenz WW , Wiegel J |
| Ref : Journal of Bacteriology , 179 :5436 , 1997 |
| Abstract : Lorenz_1997_J.Bacteriol_179_5436 |
| ESTHER : Lorenz_1997_J.Bacteriol_179_5436 |
| PubMedSearch : Lorenz_1997_J.Bacteriol_179_5436 |
| PubMedID: 9286998 |
| Gene_locus related to this paper: thesj-AXE1 |
| Title : Physical properties and kinetic behavior of a cephalosporin acetylesterase produced by Bacillus subtilis - Abbott_1975_Appl.Microbiol_30_413 |
| Author(s) : Abbott BJ , Fukuda D |
| Ref : Appl Microbiol , 30 :413 , 1975 |
| Abstract : Abbott_1975_Appl.Microbiol_30_413 |
| ESTHER : Abbott_1975_Appl.Microbiol_30_413 |
| PubMedSearch : Abbott_1975_Appl.Microbiol_30_413 |
| PubMedID: 241292 |