6-monoacetylmorphine
General
Type : Natural || Drug || Isoquinoline || Alkaloid
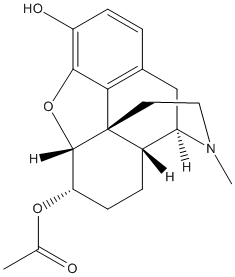
Chemical_Nomenclature : (9-hydroxy-3-methyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7-yl) acetate
Canonical SMILES : CC(=O)OC1C=CC2C3CC4=C5C2(C1OC5=C(C=C4)O)CCN3C
InChI : InChI=1S\/C19H21NO4\/c1-10(21)23-15-6-4-12-13-9-11-3-5-14(22)17-16(11)19(12,18(15)24-17)7-8-20(13)2\/h3-6,12-13,15,18,22H,7-9H2,1-2H3
InChIKey : JJGYGPZNTOPXGV-UHFFFAOYSA-N
Other name(s) : O6 Monoacetylmorphine, Monoacetylmorphine, Morphine 6-acetate, 6-O-Acetylmorphine, 6-MAM
MW : 327.38
Formula : C19H21NO4
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : BCHE
References (8)
| Title : Kinetic characterization of cholinesterases and a therapeutically valuable cocaine hydrolase for their catalytic activities against heroin and its metabolite 6-monoacetylmorphine - Kim_2018_Chem.Biol.Interact_293_107 |
| Author(s) : Kim K , Yao J , Jin Z , Zheng F , Zhan CG |
| Ref : Chemico-Biological Interactions , 293 :107 , 2018 |
| Abstract : Kim_2018_Chem.Biol.Interact_293_107 |
| ESTHER : Kim_2018_Chem.Biol.Interact_293_107 |
| PubMedSearch : Kim_2018_Chem.Biol.Interact_293_107 |
| PubMedID: 30080993 |
| Title : Possible mechanism for inhibition of morphine formation from 6-acetylmorphine after intake of street heroin - Andersson_2015_Forensic.Sci.Int_252_150 |
| Author(s) : Andersson M , Bjorkhem-Bergman L , Beck O |
| Ref : Forensic Science International , 252 :150 , 2015 |
| Abstract : Andersson_2015_Forensic.Sci.Int_252_150 |
| ESTHER : Andersson_2015_Forensic.Sci.Int_252_150 |
| PubMedSearch : Andersson_2015_Forensic.Sci.Int_252_150 |
| PubMedID: 26002801 |
| Title : Reaction pathways and free energy profiles for cholinesterase-catalyzed hydrolysis of 6-monoacetylmorphine - Qiao_2014_Org.Biomol.Chem_12_2214 |
| Author(s) : Qiao Y , Han K , Zhan CG |
| Ref : Org Biomol Chem , 12 :2214 , 2014 |
| Abstract : Qiao_2014_Org.Biomol.Chem_12_2214 |
| ESTHER : Qiao_2014_Org.Biomol.Chem_12_2214 |
| PubMedSearch : Qiao_2014_Org.Biomol.Chem_12_2214 |
| PubMedID: 24595354 |
| Title : Diacetylmorphine degradation to 6-monoacetylmorphine and morphine in cell culture: implications for in vitro studies - Hutchinson_2002_Eur.J.Pharmacol_453_27 |
| Author(s) : Hutchinson MR , Somogyi AA |
| Ref : European Journal of Pharmacology , 453 :27 , 2002 |
| Abstract : Hutchinson_2002_Eur.J.Pharmacol_453_27 |
| ESTHER : Hutchinson_2002_Eur.J.Pharmacol_453_27 |
| PubMedSearch : Hutchinson_2002_Eur.J.Pharmacol_453_27 |
| PubMedID: 12393056 |
| Title : Human erythrocyte but not brain acetylcholinesterase hydrolyses heroin to morphine - Salmon_1999_Clin.Exp.Pharmacol.Physiol_26_596 |
| Author(s) : Salmon AY , Goren Z , Avissar Y , Soreq H |
| Ref : Clinical & Experimental Pharmacology & Physiology , 26 :596 , 1999 |
| Abstract : Salmon_1999_Clin.Exp.Pharmacol.Physiol_26_596 |
| ESTHER : Salmon_1999_Clin.Exp.Pharmacol.Physiol_26_596 |
| PubMedSearch : Salmon_1999_Clin.Exp.Pharmacol.Physiol_26_596 |
| PubMedID: 10474772 |
| Title : Metabolism of cocaine and heroin is catalyzed by the same human liver carboxylesterases - Kamendulis_1996_J.Pharmacol.Exp.Ther_279_713 |
| Author(s) : Kamendulis LM , Brzezinski MR , Pindel EV , Bosron WF , Dean RA |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 279 :713 , 1996 |
| Abstract : Kamendulis_1996_J.Pharmacol.Exp.Ther_279_713 |
| ESTHER : Kamendulis_1996_J.Pharmacol.Exp.Ther_279_713 |
| PubMedSearch : Kamendulis_1996_J.Pharmacol.Exp.Ther_279_713 |
| PubMedID: 8930175 |
| Title : Alteration of in vivo and in vitro effects of heroin by esterase inhibition - Gianutsos_1986_Toxicol.Appl.Pharmacol_82_14 |
| Author(s) : Gianutsos G , Cohen SD , Carlson G , Heyman R , Salva P , Morrow G , Hite GJ |
| Ref : Toxicol Appl Pharmacol , 82 :14 , 1986 |
| Abstract : Gianutsos_1986_Toxicol.Appl.Pharmacol_82_14 |
| ESTHER : Gianutsos_1986_Toxicol.Appl.Pharmacol_82_14 |
| PubMedSearch : Gianutsos_1986_Toxicol.Appl.Pharmacol_82_14 |
| PubMedID: 3003965 |
| Title : Pharmacokinetics of morphine and its surrogates IV: Pharmacokinetics of heroin and its derived metabolites in dogs - Garrett_1980_J.Pharm.Sci_69_1116 |
| Author(s) : Garrett ER , Gurkan T |
| Ref : J Pharm Sci , 69 :1116 , 1980 |
| Abstract : Garrett_1980_J.Pharm.Sci_69_1116 |
| ESTHER : Garrett_1980_J.Pharm.Sci_69_1116 |
| PubMedSearch : Garrett_1980_J.Pharm.Sci_69_1116 |
| PubMedID: 7420276 |