5-Deoxystrigol
General
Type : Plant hormone || Strigolactone || Strigolactone receptors ligand || Natural || Inden
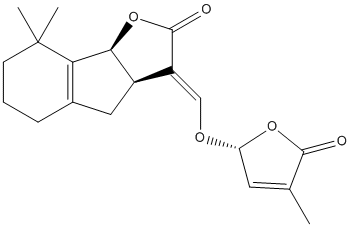
Chemical_Nomenclature : (3E,3aR,8bS)-8,8-dimethyl-3-[[(2R)-4-methyl-5-oxo-2H-furan-2-yl]oxymethylidene]-3a,4,5,6,7,8b-hexahydroindeno[1,2-b]furan-2-one
Canonical SMILES : CC1=CC(OC1=O)OC=C2C3CC4=C(C3OC2=O)C(CCC4)(C)C
InChI : InChI=1S\/C19H22O5\/c1-10-7-14(23-17(10)20)22-9-13-12-8-11-5-4-6-19(2,3)15(11)16(12)24-18(13)21\/h7,9,12,14,16H,4-6,8H2,1-3H3\/b13-9+\/t12-,14-,16+\/m1\/s1
InChIKey : QXTUQXRFEBHUBA-DYLOANJQSA-N
Other name(s) : (+)-5-deoxystrigol, SCHEMBL13857721, CHEBI:81466, C18037, 5DS, 5-DS
MW : 330.37
Formula : C19H22O5
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : RsbQ-like, Plant_carboxylesterase
References (6)
| Title : Catabolism of strigolactones by a carboxylesterase - Xu_2021_Nat.Plants_7_1495 |
| Author(s) : Xu E , Chai L , Zhang S , Yu R , Zhang X , Xu C , Hu Y |
| Ref : Nat Plants , 7 :1495 , 2021 |
| Abstract : Xu_2021_Nat.Plants_7_1495 |
| ESTHER : Xu_2021_Nat.Plants_7_1495 |
| PubMedSearch : Xu_2021_Nat.Plants_7_1495 |
| PubMedID: 34764442 |
| Gene_locus related to this paper: arath-CXE15 |
| Title : A new UHPLC-MS\/MS method for the direct determination of strigolactones in root exudates and extracts - Rial_2019_Phytochem.Anal_30_110 |
| Author(s) : Rial C , Varela RM , Molinillo JMG , Lopez-Raez JA , Macias FA |
| Ref : Phytochem Anal , 30 :110 , 2019 |
| Abstract : Rial_2019_Phytochem.Anal_30_110 |
| ESTHER : Rial_2019_Phytochem.Anal_30_110 |
| PubMedSearch : Rial_2019_Phytochem.Anal_30_110 |
| PubMedID: 30280444 |
| Title : A femtomolar-range suicide germination stimulant for the parasitic plant Striga hermonthica - Uraguchi_2018_Science_362_1301 |
| Author(s) : Uraguchi D , Kuwata K , Hijikata Y , Yamaguchi R , Imaizumi H , Am S , Rakers C , Mori N , Akiyama K , Irle S , McCourt P , Kinoshita T , Ooi T , Tsuchiya Y |
| Ref : Science , 362 :1301 , 2018 |
| Abstract : Uraguchi_2018_Science_362_1301 |
| ESTHER : Uraguchi_2018_Science_362_1301 |
| PubMedSearch : Uraguchi_2018_Science_362_1301 |
| PubMedID: 30545887 |
| Title : Strigolactone Hormones and Their Stereoisomers Signal through Two Related Receptor Proteins to Induce Different Physiological Responses in Arabidopsis - Scaffidi_2014_Plant.Physiol_165_1221 |
| Author(s) : Scaffidi A , Waters MT , Sun YK , Skelton BW , Dixon KW , Ghisalberti EL , Flematti GR , Smith SM |
| Ref : Plant Physiol , 165 :1221 , 2014 |
| Abstract : Scaffidi_2014_Plant.Physiol_165_1221 |
| ESTHER : Scaffidi_2014_Plant.Physiol_165_1221 |
| PubMedSearch : Scaffidi_2014_Plant.Physiol_165_1221 |
| PubMedID: 24808100 |
| Title : Confirming stereochemical structures of strigolactones produced by rice and tobacco - Xie_2013_Mol.Plant_6_153 |
| Author(s) : Xie X , Yoneyama K , Kisugi T , Uchida K , Ito S , Akiyama K , Hayashi H , Yokota T , Nomura T |
| Ref : Mol Plant , 6 :153 , 2013 |
| Abstract : Xie_2013_Mol.Plant_6_153 |
| ESTHER : Xie_2013_Mol.Plant_6_153 |
| PubMedSearch : Xie_2013_Mol.Plant_6_153 |
| PubMedID: 23204500 |
| Title : d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers - Arite_2009_Plant.Cell.Physiol_50_1416 |
| Author(s) : Arite T , Umehara M , Ishikawa S , Hanada A , Maekawa M , Yamaguchi S , Kyozuka J |
| Ref : Plant Cell Physiol , 50 :1416 , 2009 |
| Abstract : Arite_2009_Plant.Cell.Physiol_50_1416 |
| ESTHER : Arite_2009_Plant.Cell.Physiol_50_1416 |
| PubMedSearch : Arite_2009_Plant.Cell.Physiol_50_1416 |
| PubMedID: 19542179 |
| Gene_locus related to this paper: orysj-Q10QA5 |