4-methylumbelliferyl-heptanoate
General
Type : Coumarin
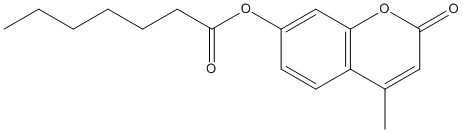
Chemical_Nomenclature : (4-methyl-2-oxochromen-7-yl) heptanoate
Canonical SMILES : CCCCCCC(=O)OC1=CC2=C(C=C1)C(=CC(=O)O2)C
InChI : InChI=1S\/C17H20O4\/c1-3-4-5-6-7-16(18)20-13-8-9-14-12(2)10-17(19)21-15(14)11-13\/h8-11H,3-7H2,1-2H3
InChIKey : FFNBFZWIBOIPIV-UHFFFAOYSA-N
Other name(s) : 4-Methylumbelliferyl heptanoate, 4-MU-Heptanoate, 4MU-Heptanoate, 4MU-C7, 4-MUH, 4-methyl-2-oxo-2h-chromen-7-yl heptanoate, 4-Methylumbelliferyl enanthate, 4-methyl-2-oxochromen-7-yl heptanoate, Heptanoic acid 4-methylumbelliferyl ester, 4-Methylumbelliferone heptanoate, 4-Methylumbelliferone-heptanoate
Target
Families : Thioesterase, Plant_carboxylesterase, A85-EsteraseD-FGH
References (5)
| Title : Structural basis for the inhibitor and substrate specificity of the unique Fph serine hydrolases of Staphylococcus aureus - Fellner_2020_ACS.Infect.Dis_6_2771 |
| Author(s) : Fellner M , Lentz CS , Jamieson SA , Brewster JL , Chen L , Bogyo M , Mace PD |
| Ref : ACS Infect Dis , 6 :2771 , 2020 |
| Abstract : Fellner_2020_ACS.Infect.Dis_6_2771 |
| ESTHER : Fellner_2020_ACS.Infect.Dis_6_2771 |
| PubMedSearch : Fellner_2020_ACS.Infect.Dis_6_2771 |
| PubMedID: 32865965 |
| Gene_locus related to this paper: staau-MW0741 , staau-MW2456 , staau-SA1143 , staau-SA2240 , staau-SA2306 , staau-SA2367 , staau-SA2422 , staau-SAV0457 , staau-SAV1793 , staau-SAV2188 |
| Title : Repositioning proton pump inhibitors as anticancer drugs by targeting the thioesterase domain of human fatty acid synthase - Fako_2015_J.Med.Chem_58_778 |
| Author(s) : Fako VE , Wu X , Pflug B , Liu JY , Zhang JT |
| Ref : Journal of Medicinal Chemistry , 58 :778 , 2015 |
| Abstract : Fako_2015_J.Med.Chem_58_778 |
| ESTHER : Fako_2015_J.Med.Chem_58_778 |
| PubMedSearch : Fako_2015_J.Med.Chem_58_778 |
| PubMedID: 25513712 |
| Title : High-resolution crystal structure of plant carboxylesterase AeCXE1, from Actinidia eriantha, and its complex with a high-affinity inhibitor paraoxon - Ileperuma_2007_Biochemistry_46_1851 |
| Author(s) : Ileperuma NR , Marshall SD , Squire CJ , Baker HM , Oakeshott JG , Russell RJ , Plummer KM , Newcomb RD , Baker EN |
| Ref : Biochemistry , 46 :1851 , 2007 |
| Abstract : Ileperuma_2007_Biochemistry_46_1851 |
| ESTHER : Ileperuma_2007_Biochemistry_46_1851 |
| PubMedSearch : Ileperuma_2007_Biochemistry_46_1851 |
| PubMedID: 17256879 |
| Gene_locus related to this paper: actde-CXE1 |
| Title : Novel antagonists of the thioesterase domain of human fatty acid synthase - Richardson_2007_Mol.Cancer.Ther_6_2120 |
| Author(s) : Richardson RD , Smith JW |
| Ref : Mol Cancer Ther , 6 :2120 , 2007 |
| Abstract : Richardson_2007_Mol.Cancer.Ther_6_2120 |
| ESTHER : Richardson_2007_Mol.Cancer.Ther_6_2120 |
| PubMedSearch : Richardson_2007_Mol.Cancer.Ther_6_2120 |
| PubMedID: 17620441 |
| Gene_locus related to this paper: human-FASN |
| Title : Fluorometric assay for the hydrolytic activity of lipase using fatty acyl esters of 4-methylumbelliferone - |
| Author(s) : Jacks TJ , Kircher HW |
| Ref : Analytical Biochemistry , 21 :279 , 1967 |
| PubMedID: 5582971 |