3-oxo-C12-HSL
General
Type : Lactone || Dodecanoate || Homoserine
Chemical_Nomenclature : 3-oxo-N-[(3S)-2-oxooxolan-3-yl]dodecanamide
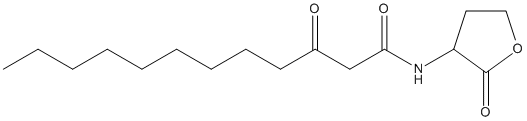
Canonical SMILES : CCCCCCCCCC(=O)CC(=O)NC1CCOC1=O
InChI : InChI=1S\/C16H27NO4\/c1-2-3-4-5-6-7-8-9-13(18)12-15(19)17-14-10-11-21-16(14)20\/h14H,2-12H2,1H3,(H,17,19)\/t14-\/m0\/s1
InChIKey : PHSRRHGYXQCRPU-AWEZNQCLSA-N
Other name(s) : N-(3-oxododecanoyl)homoserine lactone, CHEBI:44534, N-3-oxo-dodecanoyl-L-homoserine lactone, 3-oxo-C12-HSL, 3-oxo-C12-AHL
MW : 297.39
Formula : C16H27NO4
CAS_number : 168982-69-2 || 152833-54-0
PubChem :
UniChem :
Iuphar :
Target
Families : AHL-acylase
References (6)
| Title : The bacterial quorum-sensing molecule, N-3-oxo-dodecanoyl-L-homoserine lactone, inhibits mediator release and chemotaxis of murine mast cells - Khambati_2017_Inflamm.Res_66_259 |
| Author(s) : Khambati I , Han S , Pijnenburg D , Jang H , Forsythe P |
| Ref : Inflamm Res , 66 :259 , 2017 |
| Abstract : Khambati_2017_Inflamm.Res_66_259 |
| ESTHER : Khambati_2017_Inflamm.Res_66_259 |
| PubMedSearch : Khambati_2017_Inflamm.Res_66_259 |
| PubMedID: 27896412 |
| Title : High-resolution structures of AidH complexes provide insights into a novel catalytic mechanism for N-acyl homoserine lactonase - Gao_2013_Acta.Crystallogr.D.Biol.Crystallogr_69_82 |
| Author(s) : Gao A , Mei GY , Liu S , Wang P , Tang Q , Liu YP , Wen H , An XM , Zhang LQ , Yan XX , Liang DC |
| Ref : Acta Crystallographica D Biol Crystallogr , 69 :82 , 2013 |
| Abstract : Gao_2013_Acta.Crystallogr.D.Biol.Crystallogr_69_82 |
| ESTHER : Gao_2013_Acta.Crystallogr.D.Biol.Crystallogr_69_82 |
| PubMedSearch : Gao_2013_Acta.Crystallogr.D.Biol.Crystallogr_69_82 |
| PubMedID: 23275166 |
| Gene_locus related to this paper: 9rhiz-d2j2t6 |
| Title : Paraoxonases-2 and -3 Are Important Defense Enzymes against Pseudomonas aeruginosa Virulence Factors due to Their Anti-Oxidative and Anti-Inflammatory Properties - Schweikert_2012_J.Lipids_2012_352857 |
| Author(s) : Schweikert EM , Amort J , Wilgenbus P , Forstermann U , Teiber JF , Horke S |
| Ref : J Lipids , 2012 :352857 , 2012 |
| Abstract : Schweikert_2012_J.Lipids_2012_352857 |
| ESTHER : Schweikert_2012_J.Lipids_2012_352857 |
| PubMedSearch : Schweikert_2012_J.Lipids_2012_352857 |
| PubMedID: 22570791 |
| Title : Inactivation of AHLs by Ochrobactrum sp. A44 depends on the activity of a novel class of AHL acylase - Czajkowski_2011_Environ.Microbiol.Rep_3_59 |
| Author(s) : Czajkowski R , Krzyzanowska D , Karczewska J , Atkinson S , Przysowa J , Lojkowska E , Williams P , Jafra S |
| Ref : Environ Microbiol Rep , 3 :59 , 2011 |
| Abstract : Czajkowski_2011_Environ.Microbiol.Rep_3_59 |
| ESTHER : Czajkowski_2011_Environ.Microbiol.Rep_3_59 |
| PubMedSearch : Czajkowski_2011_Environ.Microbiol.Rep_3_59 |
| PubMedID: 23761232 |
| Gene_locus related to this paper: 9rhiz-d8x182 |
| Title : Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. - Czajkowski_2009_Acta.Biochim.Pol_56_1 |
| Author(s) : Czajkowski R , Jafra S |
| Ref : Acta Biochim Pol , 56 :1 , 2009 |
| Abstract : Czajkowski_2009_Acta.Biochim.Pol_56_1 |
| ESTHER : Czajkowski_2009_Acta.Biochim.Pol_56_1 |
| PubMedSearch : Czajkowski_2009_Acta.Biochim.Pol_56_1 |
| PubMedID: 19287806 |
| Title : Influence of Pseudomonas aeruginosa quorum sensing signal molecule N-(3-oxododecanoyl) homoserine lactone on mast cells - Li_2009_Med.Microbiol.Immunol_198_113 |
| Author(s) : Li H , Wang L , Ye L , Mao Y , Xie X , Xia C , Chen J , Lu Z , Song J |
| Ref : Med Microbiol Immunol , 198 :113 , 2009 |
| Abstract : Li_2009_Med.Microbiol.Immunol_198_113 |
| ESTHER : Li_2009_Med.Microbiol.Immunol_198_113 |
| PubMedSearch : Li_2009_Med.Microbiol.Immunol_198_113 |
| PubMedID: 19337750 |