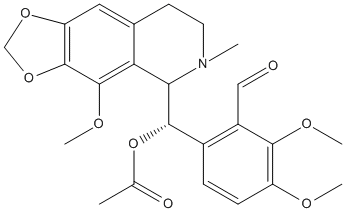
3-O-Acetylpapaveroxine
General
Type : Isoquinoline || Benzodioxo || Acetate
Chemical_Nomenclature : [(S)-(2-formyl-3,4-dimethoxyphenyl)-[(5R)-4-methoxy-6-methyl-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]isoquinolin-5-yl]methyl] acetate
Canonical SMILES : CC(=O)OC(C1C2=C(C3=C(C=C2CCN1C)OCO3)OC)C4=C(C(=C(C=C4)OC)OC)C=O
InChI : InChI=1S\/C24H27NO8\/c1-13(27)33-22(15-6-7-17(28-3)21(29-4)16(15)11-26)20-19-14(8-9-25(20)2)10-18-23(24(19)30-5)32-12-31-18\/h6-7,10-11,20,22H,8-9,12H2,1-5H3\/t20-,22+\/m1\/s1
InChIKey : JUUOWVIBIPDOSQ-IRLDBZIGSA-N
Other name(s) : C21591
MW : 457.47
Formula : C24H27NO8
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Plant_carboxylesterase
References (2)
| Title : Family portraits: the enzymes behind benzylisoquinoline alkaloid diversity - Dastmalchi_2018_Phytochem.Rev_17_249 |
| Author(s) : Dastmalchi M , Park MR , Morris JS , Facchini P |
| Ref : Phytochemistry Review , 17 :249 , 2018 |
| Abstract : Dastmalchi_2018_Phytochem.Rev_17_249 |
| ESTHER : Dastmalchi_2018_Phytochem.Rev_17_249 |
| PubMedSearch : Dastmalchi_2018_Phytochem.Rev_17_249 |
| PubMedID: |
| Title : Acetylation serves as a protective group in noscapine biosynthesis in opium poppy - Dang_2015_Nat.Chem.Biol_11_104 |
| Author(s) : Dang TT , Chen X , Facchini PJ |
| Ref : Nat Chemical Biology , 11 :104 , 2015 |
| Abstract : Dang_2015_Nat.Chem.Biol_11_104 |
| ESTHER : Dang_2015_Nat.Chem.Biol_11_104 |
| PubMedSearch : Dang_2015_Nat.Chem.Biol_11_104 |
| PubMedID: 25485687 |
| Gene_locus related to this paper: papso-cxe2 , papso-cxe1 |