2,4-D-Methyl
General
Type : Herbicide || Not A\/B H target
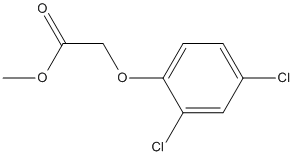
Chemical_Nomenclature : methyl 2-(2,4-dichlorophenoxy)acetate
Canonical SMILES : COC(=O)COC1=C(C=C(C=C1)Cl)Cl
InChI : InChI=1S\/C9H8Cl2O3\/c1-13-9(12)5-14-8-3-2-6(10)4-7(8)11\/h2-4H,5H2,1H3
InChIKey : HWIGZMADSFQMOI-UHFFFAOYSA-N
Other name(s) : 2,4-D methyl ester, Methyl (2,4-dichlorophenoxy)acetate, methyl 2-(2,4-dichlorophenoxy)acetate, SCHEMBL5004223, CHEMBL1327631, ZINC57288
Target
Families : Plant_carboxylesterase
References (2)
| Title : Role of a carboxylesterase in herbicide bioactivation in Arabidopsis thaliana - Gershater_2007_J.Biol.Chem_282_21460 |
| Author(s) : Gershater MC , Cummins I , Edwards R |
| Ref : Journal of Biological Chemistry , 282 :21460 , 2007 |
| Abstract : Gershater_2007_J.Biol.Chem_282_21460 |
| ESTHER : Gershater_2007_J.Biol.Chem_282_21460 |
| PubMedSearch : Gershater_2007_J.Biol.Chem_282_21460 |
| PubMedID: 17519238 |
| Gene_locus related to this paper: arath-CXE12 |
| Title : Carboxylesterase activities toward pesticide esters in crops and weeds - Gershater_2006_Phytochemistry_67_2561 |
| Author(s) : Gershater M , Sharples K , Edwards R |
| Ref : Phytochemistry , 67 :2561 , 2006 |
| Abstract : Gershater_2006_Phytochemistry_67_2561 |
| ESTHER : Gershater_2006_Phytochemistry_67_2561 |
| PubMedSearch : Gershater_2006_Phytochemistry_67_2561 |
| PubMedID: 17078983 |