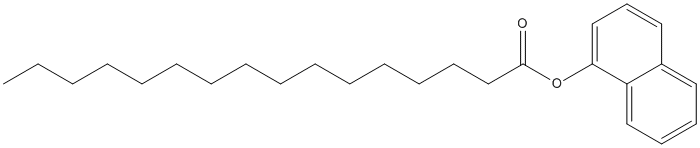
1-Naphtylpalmitate
General
Type : Palmitate || Naphtyl ester || Aromatic ester (two rings) || Hexadecanoate || Naphthalen
Chemical_Nomenclature : naphthalen-1-yl hexadecanoate
Canonical SMILES : CCCCCCCCCCCCCCCC(=O)OC1=CC=CC2=CC=CC=C21
InChI : InChI=1S\/C26H38O2\/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-22-26(27)28-25-21-17-19-23-18-15-16-20-24(23)25\/h15-21H,2-14,22H2,1H3
InChIKey : IAKVKXNIMLQHPA-UHFFFAOYSA-N
Other name(s) : alpha-naphtyl palmitate, Naphthyl palmitate, alpha-Naphthylpalmitate, 1-naphthyl palmitate, C16-naphthyl ester, SCHEMBL8015856, ZINC59681574
Target
Families : Tannase, Bacterial_lip_FamI.1, Fungal-Bact_LIP
References (3)
| Title : Dieselzymes: development of a stable and methanol tolerant lipase for biodiesel production by directed evolution. - Korman_2013_Biotechnol.Biofuels_6_70 |
| Author(s) : Korman TP , Sahachartsiri B , Charbonneau DM , Huang GL , Beauregard M , Bowie JU |
| Ref : Biotechnol Biofuels , 6 :70 , 2013 |
| Abstract : Korman_2013_Biotechnol.Biofuels_6_70 |
| ESTHER : Korman_2013_Biotechnol.Biofuels_6_70 |
| PubMedSearch : Korman_2013_Biotechnol.Biofuels_6_70 |
| PubMedID: 23648063 |
| Gene_locus related to this paper: promi-c2lfd0 |
| Title : Characterization of a novel lipolytic enzyme from Aspergillus oryzae - Koseki_2013_Appl.Microbiol.Biotechnol_97_5351 |
| Author(s) : Koseki T , Asai S , Saito N , Mori M , Sakaguchi Y , Ikeda K , Shiono Y |
| Ref : Applied Microbiology & Biotechnology , 97 :5351 , 2013 |
| Abstract : Koseki_2013_Appl.Microbiol.Biotechnol_97_5351 |
| ESTHER : Koseki_2013_Appl.Microbiol.Biotechnol_97_5351 |
| PubMedSearch : Koseki_2013_Appl.Microbiol.Biotechnol_97_5351 |
| PubMedID: 23001008 |
| Gene_locus related to this paper: aspor-q2uf27 |
| Title : Purification and characterization of a secretory lipolytic enzyme, MgLIP2, from Malassezia globosa - Juntachai_2011_Microbiol.(Reading)_157_3492 |
| Author(s) : Juntachai W , Oura T , Kajiwara S |
| Ref : Microbiology (Reading) , 157 :3492 , 2011 |
| Abstract : Juntachai_2011_Microbiol.(Reading)_157_3492 |
| ESTHER : Juntachai_2011_Microbiol.(Reading)_157_3492 |
| PubMedSearch : Juntachai_2011_Microbiol.(Reading)_157_3492 |
| PubMedID: 22016565 |
| Gene_locus related to this paper: malgo-a8qcw4 |