1,3-Diolein
General
Type : Acyl-Glycerol || Diacylglycerol || Fatty acid ester || Water-insoluble medium and long chain ester || Oleate
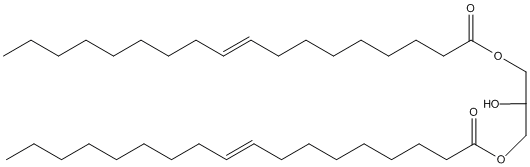
Chemical_Nomenclature : [2-hydroxy-3-[(Z)-octadec-9-enoyl]oxypropyl] (Z)-octadec-9-enoate
Canonical SMILES : CCCCCCCCC=CCCCCCCCC(=O)OCC(COC(=O)CCCCCCCC=CCCCCCCCC)O
InChI : InChI=1S\/C39H72O5\/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-38(41)43-35-37(40)36-44-39(42)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2\/h17-20,37,40H,3-16,21-36H2,1-2H3\/b19-17-,20-18-
InChIKey : DRAWQKGUORNASA-CLFAGFIQSA-N
Other name(s) : 1,3-Dioleoylglycerol, Glycerol 1,3-dioleate, 2-Hydroxy-1,3-propanediyl dioleate, Diacylglycerol(18:1\/0:0\/18:1)
Target
Families : Canar_LipB
References (3)
| Title : Rational synthesis of 1,3-diolein by enzymatic esterification - Duan_2012_J.Biotechnol_159_44 |
| Author(s) : Duan ZQ , Du W , Liu DH |
| Ref : J Biotechnol , 159 :44 , 2012 |
| Abstract : Duan_2012_J.Biotechnol_159_44 |
| ESTHER : Duan_2012_J.Biotechnol_159_44 |
| PubMedSearch : Duan_2012_J.Biotechnol_159_44 |
| PubMedID: 22370538 |
| Title : The mechanism of solvent effect on the positional selectivity of Candida antarctica lipase B during 1,3-diolein synthesis by esterification - Duan_2011_Bioresour.Technol_102_11048 |
| Author(s) : Duan ZQ , Du W , Liu DH |
| Ref : Bioresour Technol , 102 :11048 , 2011 |
| Abstract : Duan_2011_Bioresour.Technol_102_11048 |
| ESTHER : Duan_2011_Bioresour.Technol_102_11048 |
| PubMedSearch : Duan_2011_Bioresour.Technol_102_11048 |
| PubMedID: 21978621 |
| Title : The solvent influence on the positional selectivity of Novozym 435 during 1,3-diolein synthesis by esterification - Duan_2010_Bioresour.Technol_101_2568 |
| Author(s) : Duan ZQ , Du W , Liu DH |
| Ref : Bioresour Technol , 101 :2568 , 2010 |
| Abstract : Duan_2010_Bioresour.Technol_101_2568 |
| ESTHER : Duan_2010_Bioresour.Technol_101_2568 |
| PubMedSearch : Duan_2010_Bioresour.Technol_101_2568 |
| PubMedID: 20022242 |