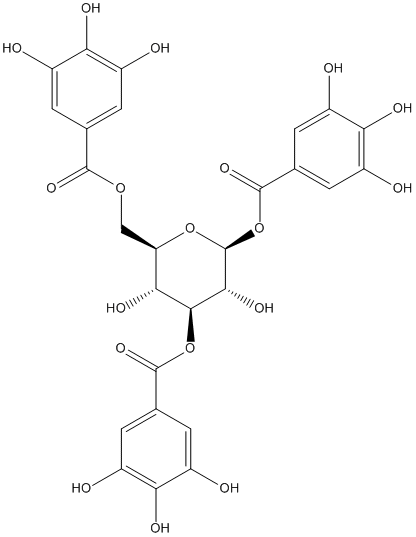
1,3,6-Trigalloylglucose
General
Type : Derivative of Gallate || Natural || Polyphenol
Chemical_Nomenclature :
Canonical SMILES :
InChI :
InChIKey :
Other name(s) : 1,3,6-Trigalloyl glucose, 1,3,6-Tri-O-galloyl-b-D-glucose, 1,3,6-tri-O-galloyl-beta-D-glucose, 1,3,6-tri-o-galloylglucose, 1,3,6-Tri-O-galloyl-b-D-glucopyranose, CHEMBL389895, SCHEMBL1004538
Target
Families : Tannase, Tannase_Bact
References (13)
| Title : New insights into the function of plant tannase with promiscuous acyltransferase activity - Chen_2022_Plant.J__ |
| Author(s) : Chen Y , Jiang C , Yin S , Zhuang J , Zhao Y , Zhang L , Jiang X , Liu Y , Gao L , Xia T |
| Ref : Plant J , : , 2022 |
| Abstract : Chen_2022_Plant.J__ |
| ESTHER : Chen_2022_Plant.J__ |
| PubMedSearch : Chen_2022_Plant.J__ |
| PubMedID: 36534122 |
| Gene_locus related to this paper: camsi-CsTA |
| Title : A structurally unique Fusobacterium nucleatum tannase provides detoxicant activity against gallotannins and pathogen resistance - Mancheno_2022_Microb.Biotechnol_15_648 |
| Author(s) : Mancheno JM , Atondo E , Tomas-Cortazar J , Luis Lavin J , Plaza-Vinuesa L , Martin-Ruiz I , Barriales D , Palacios A , Daniel Navo C , Sampedro L , Pena-Cearra A , Angel Pascual-Itoiz M , Castelo J , Carreras-Gonzalez A , Castellana D , Pellon A , Delgado S , Ruas-Madiedo P , de Las Rivas B , Abecia L , Munoz R , Jimenez-Oses G , Anguita J , Rodriguez H |
| Ref : Microb Biotechnol , 15 :648 , 2022 |
| Abstract : Mancheno_2022_Microb.Biotechnol_15_648 |
| ESTHER : Mancheno_2022_Microb.Biotechnol_15_648 |
| PubMedSearch : Mancheno_2022_Microb.Biotechnol_15_648 |
| PubMedID: 33336898 |
| Gene_locus related to this paper: fusnn-FN0616 |
| Title : Structural diversity and substrate preferences of three tannase enzymes encoded by the anaerobic bacterium Clostridium butyricum - Ristinmaa_2022_J.Biol.Chem_298_101758 |
| Author(s) : Ristinmaa AS , Coleman T , Cesar L , Langborg Weinmann A , Mazurkewich S , Branden G , Hasani M , Larsbrink J |
| Ref : Journal of Biological Chemistry , 298 :101758 , 2022 |
| Abstract : Ristinmaa_2022_J.Biol.Chem_298_101758 |
| ESTHER : Ristinmaa_2022_J.Biol.Chem_298_101758 |
| PubMedSearch : Ristinmaa_2022_J.Biol.Chem_298_101758 |
| PubMedID: 35202648 |
| Gene_locus related to this paper: clobu-CbTan2 |
| Title : Crystal structure of fungal tannase from Aspergillus niger - Dong_2021_Acta.Crystallogr.D.Struct.Biol_77_267 |
| Author(s) : Dong L , McKinstry WJ , Pan L , Newman J , Ren B |
| Ref : Acta Crystallographica D Struct Biol , 77 :267 , 2021 |
| Abstract : Dong_2021_Acta.Crystallogr.D.Struct.Biol_77_267 |
| ESTHER : Dong_2021_Acta.Crystallogr.D.Struct.Biol_77_267 |
| PubMedSearch : Dong_2021_Acta.Crystallogr.D.Struct.Biol_77_267 |
| PubMedID: 33559614 |
| Gene_locus related to this paper: aspnc-a2qir3 |
| Title : Exploring the Degradation of Gallotannins Catalyzed by Tannase Produced by Aspergillus niger GH1 for Ellagic Acid Production in Submerged and Solid-State Fermentation - Chavez-Gonzalez_2018_Appl.Biochem.Biotechnol_185_476 |
| Author(s) : Chavez-Gonzalez ML , Guyot S , Rodriguez-Herrera R , Prado-Barragan A , Aguilar CN |
| Ref : Appl Biochem Biotechnol , 185 :476 , 2018 |
| Abstract : Chavez-Gonzalez_2018_Appl.Biochem.Biotechnol_185_476 |
| ESTHER : Chavez-Gonzalez_2018_Appl.Biochem.Biotechnol_185_476 |
| PubMedSearch : Chavez-Gonzalez_2018_Appl.Biochem.Biotechnol_185_476 |
| PubMedID: 29181764 |
| Title : Gallic acid production under anaerobic submerged fermentation by two bacilli strains - Aguilar-Zarate_2015_Microb.Cell.Fact_14_209 |
| Author(s) : Aguilar-Zarate P , Cruz MA , Montanez J , Rodriguez-Herrera R , Wong-Paz JE , Belmares RE , Aguilar CN |
| Ref : Microb Cell Fact , 14 :209 , 2015 |
| Abstract : Aguilar-Zarate_2015_Microb.Cell.Fact_14_209 |
| ESTHER : Aguilar-Zarate_2015_Microb.Cell.Fact_14_209 |
| PubMedSearch : Aguilar-Zarate_2015_Microb.Cell.Fact_14_209 |
| PubMedID: 26715179 |
| Title : Genetic and biochemical approaches towards unravelling the degradation of gallotannins by Streptococcus gallolyticus - Jimenez_2014_Microb.Cell.Fact_13_154 |
| Author(s) : Jimenez N , Reveron I , Esteban-Torres M , Lopez de Felipe F , de Las Rivas B , Munoz R |
| Ref : Microb Cell Fact , 13 :154 , 2014 |
| Abstract : Jimenez_2014_Microb.Cell.Fact_13_154 |
| ESTHER : Jimenez_2014_Microb.Cell.Fact_13_154 |
| PubMedSearch : Jimenez_2014_Microb.Cell.Fact_13_154 |
| PubMedID: 25359406 |
| Gene_locus related to this paper: strg3-d3hd15 , strg3-d3hey7 |
| Title : Tannin degradation by a novel tannase enzyme present in some Lactobacillus plantarum strains - Jimenez_2014_Appl.Environ.Microbiol_80_2991 |
| Author(s) : Jimenez N , Esteban-Torres M , Mancheno JM , de Las Rivas B , Munoz R |
| Ref : Applied Environmental Microbiology , 80 :2991 , 2014 |
| Abstract : Jimenez_2014_Appl.Environ.Microbiol_80_2991 |
| ESTHER : Jimenez_2014_Appl.Environ.Microbiol_80_2991 |
| PubMedSearch : Jimenez_2014_Appl.Environ.Microbiol_80_2991 |
| PubMedID: 24610854 |
| Gene_locus related to this paper: lacpn-d7vbf4 |
| Title : Crystal structure of tannase from lactobacillusplantarum - Ren_2013_J.Mol.Biol_425_2737 |
| Author(s) : Ren B , Wu M , Wang Q , Peng X , Wen H , McKinstry WJ , Chen Q |
| Ref : Journal of Molecular Biology , 425 :2737 , 2013 |
| Abstract : Ren_2013_J.Mol.Biol_425_2737 |
| ESTHER : Ren_2013_J.Mol.Biol_425_2737 |
| PubMedSearch : Ren_2013_J.Mol.Biol_425_2737 |
| PubMedID: 23648840 |
| Gene_locus related to this paper: lacpl-tanL |
| Title : Recent advances in industrial application of tannases: a review - Beniwal_2013_Recent.Pat.Biotechnol_7_228 |
| Author(s) : Beniwal V , Kumar A , Sharma J , Chhokar V |
| Ref : Recent Pat Biotechnol , 7 :228 , 2013 |
| Abstract : Beniwal_2013_Recent.Pat.Biotechnol_7_228 |
| ESTHER : Beniwal_2013_Recent.Pat.Biotechnol_7_228 |
| PubMedSearch : Beniwal_2013_Recent.Pat.Biotechnol_7_228 |
| PubMedID: 24182319 |
| Title : Large-scale production of tannase using the yeast Arxula adeninivorans - Boer_2011_Appl.Microbiol.Biotechnol_92_105 |
| Author(s) : Boer E , Breuer FS , Weniger M , Denter S , Piontek M , Kunze G |
| Ref : Applied Microbiology & Biotechnology , 92 :105 , 2011 |
| Abstract : Boer_2011_Appl.Microbiol.Biotechnol_92_105 |
| ESTHER : Boer_2011_Appl.Microbiol.Biotechnol_92_105 |
| PubMedSearch : Boer_2011_Appl.Microbiol.Biotechnol_92_105 |
| PubMedID: 21559827 |
| Gene_locus related to this paper: blaad-b7vfd0 |
| Title : Biodegradation of gallotannins and ellagitannins - Li_2006_J.Basic.Microbiol_46_68 |
| Author(s) : Li M , Kai Y , Qiang H , Dongying J |
| Ref : J Basic Microbiol , 46 :68 , 2006 |
| Abstract : Li_2006_J.Basic.Microbiol_46_68 |
| ESTHER : Li_2006_J.Basic.Microbiol_46_68 |
| PubMedSearch : Li_2006_J.Basic.Microbiol_46_68 |
| PubMedID: 16463321 |
| Title : Secretion, purification, and characterization of a recombinant Aspergillus oryzae tannase in Pichia pastoris - Zhong_2004_Protein.Expr.Purif_36_165 |
| Author(s) : Zhong X , Peng L , Zheng S , Sun Z , Ren Y , Dong M , Xu A |
| Ref : Protein Expr Purif , 36 :165 , 2004 |
| Abstract : Zhong_2004_Protein.Expr.Purif_36_165 |
| ESTHER : Zhong_2004_Protein.Expr.Purif_36_165 |
| PubMedSearch : Zhong_2004_Protein.Expr.Purif_36_165 |
| PubMedID: 15249037 |
| Gene_locus related to this paper: aspor-tan |