1,2-epoxyoctane
General
Type : Epoxide
Chemical_Nomenclature : 2-hexyloxirane
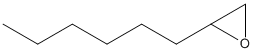
Canonical SMILES : CCCCCCC1CO1
InChI : InChI=1S\/C8H16O\/c1-2-3-4-5-6-8-7-9-8\/h8H,2-7H2,1H3
InChIKey : NJWSNNWLBMSXQR-UHFFFAOYSA-N || NJWSNNWLBMSXQR-MRVPVSSYSA-N || NJWSNNWLBMSXQR-QMMMGPOBSA-N
Other name(s) : R-EpO, S-EpO, rac-EpO, 2-Hexyloxirane, Hexyloxirane, S-1,2-epoxyoctane, S-2-hexyloxirane, 1-Octene oxide, Octylene epoxide, (2R)-2-hexyloxirane, (R)-(+)-1,2-Epoxyoctane, (R)-1,2-epoxyoctane, (R)-2-hexyloxirane, AC1LUY1G, (2S)-2-hexyloxirane, (S)-(-)-1,2-Epoxyoctane, 2beta-Hexyloxirane, (S)-2-hexyloxirane, (s)-1,2-epoxyoctane
MW : 128.21
Formula : C8H16O
CAS_number : 2984-50-1 || 77495-66-0 || 50418-68-3
PubChem :
UniChem :
Iuphar :
Target
Families : Epoxide_hydrolase, CFTR-inhibitory-factor_Cif
References (3)
| Title : Active-Site Flexibility and Substrate Specificity in a Bacterial Virulence Factor: Crystallographic Snapshots of an Epoxide Hydrolase - Hvorecny_2017_Structure_25_697 |
| Author(s) : Hvorecny KL , Bahl CD , Kitamura S , Lee KSS , Hammock BD , Morisseau C , Madden DR |
| Ref : Structure , 25 :697 , 2017 |
| Abstract : Hvorecny_2017_Structure_25_697 |
| ESTHER : Hvorecny_2017_Structure_25_697 |
| PubMedSearch : Hvorecny_2017_Structure_25_697 |
| PubMedID: 28392259 |
| Gene_locus related to this paper: pseae-PA2934 |
| Title : Ylehd, an epoxide hydrolase with promiscuous haloalkane dehalogenase activity from tropical marine yeast Yarrowia lipolytica is induced upon xenobiotic stress - Bendigiri_2017_Sci.Rep_7_11887 |
| Author(s) : Bendigiri C , Zinjarde S , Ravikumar A |
| Ref : Sci Rep , 7 :11887 , 2017 |
| Abstract : Bendigiri_2017_Sci.Rep_7_11887 |
| ESTHER : Bendigiri_2017_Sci.Rep_7_11887 |
| PubMedSearch : Bendigiri_2017_Sci.Rep_7_11887 |
| PubMedID: 28928379 |
| Gene_locus related to this paper: yarli-q6c598 |
| Title : Cloning and characterization of an epoxide hydrolase from Cupriavidus metallidurans-CH34 - Kumar_2011_Protein.Expr.Purif_79_49 |
| Author(s) : Kumar R , Wani SI , Chauhan NS , Sharma R , Sareen D |
| Ref : Protein Expr Purif , 79 :49 , 2011 |
| Abstract : Kumar_2011_Protein.Expr.Purif_79_49 |
| ESTHER : Kumar_2011_Protein.Expr.Purif_79_49 |
| PubMedSearch : Kumar_2011_Protein.Expr.Purif_79_49 |
| PubMedID: 21515382 |